Megalaspis Cordyla Linnaeus, 1758) in the Java Sea, Indonesia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Data Collection and Size Sampling on Neritic Tuna Fisheries in Andaman
IOTC–2018–WPNT08–15 Data Collection and size sampling on Neritic Tuna Fisheries in Andaman Sea Kanokwan Maeroh, Sichon Hoimuk, Suchart Inthong, and Supachai Rodpradit Upper Andaman Sea Fisheries Research and Development Center (Phuket) 77 Moo 7 Vichit Sub-District, Muang District, Phuket Province 83000 Tel. 0 7639 1138-40 e-mail: [email protected] Abstract In the Andaman Sea Coast of Thailand, there are many kinds of fishing gears can catch neritic tuna but most of it were caught by purse seine. The other gears are Otter board trawl, Anchovy falling nets and Squid Falling nets. There are 3 organizations along Andaman sea under Marine Fisheries Research and Development Division responsible to collect the data on fish composition and size distribution, especially for neritic tuna and others importantly economic fish, from more than 10 types of fishing gears. All kind of fishing gears in which both commercial and artisanal fisheries were conducted fisheries data for 3-5 days a month. There are 7 organizations along Andaman sea under Fishing and Fleets Management Division responsible to collected fishing data from logbook and catches landing of marine fish, to recorded and reported the data to Fishing-Info data base. Study on CPUE and MSY were conducted by Fisheries Statistics Analysis and Research Group and Fisheries Resources Assessment Group which are under the Department of Fishery. The purse seine is the mainly fishing gear for pelagic species. Pair trawls and otter board trawls are the main fishing gears for demersal species. The other gears which have a specific name to target species, such as gill nets, hand line and long line. -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

Surat Thani Blue Swimming Crab Fishery Improvement Project
Surat Thani Blue Swimming Crab Fishery Improvement Project -------------------------------------------------------------------------------------------------------------------------------------- Milestone 33b: Final report of bycatch research Progress report: The study of fishery biology, socio-economic and ecosystem related to the restoration of Blue Swimming Crab following Fishery improvement program (FIP) in Bandon Bay, Surat Thani province. Amornsak Sawusdee1 (1) The Center of Academic Service, Walailak University, Tha Sala, Nakhon Si Thammarat, 80160 The results of observation of catching BSC by using collapsible crab trap and floating seine. According to the observation of aquatic animal which has been caught by main BSC fishing gears; floating seine and collapsible crab trap, there were 176 kind of aquatic animals. The catch aquatic animals are shown in the table1. In this study, aquatic animal was classified into 11 Groups; Blue Swimming Crab (Portunus Pelagicus), Coelenterata (coral animals, true jellies, sea anemones, sea pens), Helcionelloida (clam, bivalve, gastropod), Cephalopoda (sqiud, octopus), Chelicerata (horseshoe crab), Hoplocari(stomatopods), Decapod (shrimp), Anomura (hermit crab), Brachyura (crab), Echinoderm (sea cucambers, sea stars, sea urchins), Vertebrata (fish). Vertebrata was the main group that was captured by BSC fishing gears, more than 70 species. Next are Helcionelloida and Helcionelloida 38 species and 29 species respectively. The sample that has been classified were photographed and attached in appendix 1. However, some species were classified as unknow which are under the classification process and reconcile. There were 89 species that were captured by floating seine. The 3 main group that were captured by this fishing gear are Vertebrata (34 species), Brachyura (20 species) Helcionelloida and Echinoderm (10 Species). On the other hand, there were 129 species that were captured by collapsible crab trap. -

Juveniles of the Torpedo Scad, Megalaspis Cordyla
NOTE UPDATE AND CORRECTION Juveniles of the Torpedo Scad, Megalaspis cordyla (Teleostei: Carangidae), schooling with venomous catfishes (Plotosidae): a new case of mimicry and an identification correction WILLIAM F. SMITH-VANIZ Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA E-mail: [email protected] Key words: fishes, ichthyology, Batesian mimicry, ecology, symbiosis, mutualism, coral reefs, Indonesia, Indian Ocean, Indo-Pacific. Citation: Smith-Vaniz, W.F. (2018) Juveniles of the Torpedo Scad, Megalaspis cordyla (Teleostei: Carangidae), schooling with venomous catfishes (Plotosidae): a new case of mimicry and an identification correction. Journal of the Ocean Science Foundation, 30, 105–107. doi: http://dx.doi.org/10.5281/zenodo.1467454 Smith-Vaniz et al. (2018) reported a case of mimicry between juveniles of Caranx bucculentus Alleyne & Macleay (Carangidae) and Plotosus lineatus (Thunberg) (Plotosidae) in this volume of the Journal of the Ocean Science Foundation (p. 82). The identifications were based solely on photographs of schooling juveniles at Lembeh Strait, Indonesia. Soon after the on-line version of the paper became available, new information made it obvious that the carangid had been misidentified. A color image of an approximately 5 cm fork length juvenile of C. bucculentus from Western Australia was kindly sent to me by John Pogonoski. This specimen has a much deeper body than the mimic carangid and its coloration is very different. Subsequently, I received 4 color photographs of what was clearly the same carangid illustrated in Smith-Vaniz et al. (2018) in association with Plotosus schools. Journal of the Ocean Science Foundation, 30, 105–107 (2018) 105 One of these photographs (Fig. -
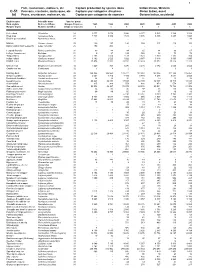
Fish, Crustaceans, Molluscs, Etc Capture
1 Fish, crustaceans, molluscs, etc Capture production by species items Indian Ocean, Western C-51 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Océan Indien, ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Océano Indico, occidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 1998 1999 2000 2001 2002 2003 2004 Nombre inglés Nombre científico Grupo de especies t t t t t t t Kelee shad Hilsa kelee 24 3 077 3 076 3 896 4 277 5 525 2 306 3 208 Hilsa shad Tenualosa ilisha 24 7 747 6 482 4 545 3 846 6 146 8 257 4 024 Bloch's gizzard shad Nematalosa nasus 24 ... ... ... ... ... ... 93 Milkfish Chanos chanos 25 139 137 125 154 117 113 101 Barramundi(=Giant seaperch) Lates calcarifer 25 196 204 - - - - - Leopard flounder Bothus pantherinus 31 90 84 84 65 84 96 67 Lefteye flounders nei Bothidae 31 9 27 129 125 88 73 78 Tonguefishes Cynoglossidae 31 2 158 2 066 2 137 1 923 1 989 1 383 984 Indian halibut Psettodes erumei 31 3 192 2 520 3 022 2 957 3 118 3 365 4 095 Flatfishes nei Pleuronectiformes 31 17 474 12 851 24 720 11 489 15 573 15 924 11 325 Unicorn cod Bregmaceros mcclellandi 32 1 449 743 1 470 2 435 2 372 2 643 2 624 Gadiformes nei Gadiformes 32 7 5 2 1 - 1 3 Bombay-duck Harpadon nehereus 33 144 865 146 663 133 221 141 082 100 366 101 936 116 452 Greater lizardfish Saurida tumbil 33 6 064 3 710 3 485 3 894 4 247 3 634 2 655 Brushtooth lizardfish Saurida undosquamis 33 34 20 30 32 20 11 18 Lizardfishes nei Synodontidae 33 20 221 20 871 17 894 12 433 12 447 10 715 13 833 Giant catfish Arius thalassinus 33 315 309 554 480 470 574 597 Sea catfishes nei Ariidae 33 99 932 98 467 78 375 89 308 89 941 78 788 81 972 Sabre squirrelfish Sargocentron spiniferum 33 34 53 46 97 96 135 114 Flathead grey mullet Mugil cephalus 33 69 89 60 34 37 40 46 Klunzinger's mullet Liza klunzingeri 33 .. -

3. MARINE FISHERY RESOURCES the Coastal Waters of Cambodia
3. MARINE FISHERY RESOURCES The coastal waters of Cambodia support a large number of marine fish and invertebrate species. Try (2003) gives the scientific, English and Kmer names for those marine species recorded from the country: 476 species of marine finfish, 20 species of marine crabs, 42 species of marine gastropods and 24 species of marine bivalves. In the marine fisheries statistics published by the Department of Fisheries (DoF, 2002), nine different groups are given. The 2001 landings of these groups by province/municipality are provided in Table 2 below. Little quantitative information is available on the composition of the finfish component. Table 2 Marine fishery landings recorded by DoF, 2001 Province Krill Krill Ray Sea Fish Crab Shrimp Total Snail Trash fish cucumber Cephalopod Blood cockle Slipper lobster Kampot 2 703 1 786 284 165 247 0 870 176 199 0 0 6 430 Sihanouk 6 943 4 287 1 730 0 1 496 40 897 1 236 226 210 0 17 065 Koh 7 104 4 764 1 606 42 604 0 1 410 1 082 762 0 26 17 400 Kong Kep 123 10 42 2 8 0 285 0 0 470 123 1 063 Total 16 873 10 847 3 662 209 2 355 40 3 462 2 494 1 187 680 149 41 958 Source: DoF, 2002. These recorded 2001 landings are depicted in Figure 2 below. It can be seen that about two- thirds of the catch consists of “fish” and “trash fish”. Figure 2. Marine fishery landings recorded by DoF, 2001 Fish Trash fish Shrimp Other Cephalopod Crab Snail Cockle Source: Table 2. -

Purse Seine Fisheries and CPUE of Neritic Tuna Fisheries in the Andaman Sea, Coast of Thailand, 2018
IOTC-2019-WPNT09-18 Purse Seine Fisheries and CPUE of Neritic Tuna Fisheries in the Andaman Sea, Coast of Thailand, 2018. Kanokwan Maeroh, Sichon Hoimuk, Pornanan Keereerat, and Noppadon Somkliang, Upper Andaman Sea Fisheries Research and Development Center (Phuket) 77 Tumbon Vichit, Maung District, Phuket 83000 Thailand Abstract Purse Seine fishery in the Andaman Sea operation was 1-2 days per trip or 21 days per month. Fishing grounds located along the Andaman Sea where distances from shores are 10 to 30 nautical miles and depth of water range from 20-80 m. The operate net made from black nylon with mesh size as 2.5 cm. Average annual catch rate from January to December 2018 was 2 ,306.5 kg/day. The peak of CPUE occurred in March to May. Catch composed of Decapterus macrosoma 15.1%, followed by Rastrelliger kanagurta 13.2%, Decapterus maruadsi 10.9%, Neritic tuna 10.1%, Selar crumenophthalmus 8.7% , Sardinella gibbosa 3.9%, Megalaspis cordyla 3.5%, Siganus canaliculatus 3.1% and other species 31.5%. The average annual catch rate of Neritic tuna was 188.6 kg/day. Neritic tuna in the Andaman Sea was caught mainly from purse seine fishery, by Thai purse seine (TPS) 825.0 kg/day (38.5%), followed by light luring purse seine (LPS) 161.0 kg/day (9.5%), and purse seine with fish aggregating devices (FADs) 148.8 kg/day (7.9%). The highest CPUE was Euthynnus affinis, Thunnus tonggol, Auxis thazard, and Auxis rochei was 66.6, 64.9, 33.0 and 24.1 kg/day, respectively. -

Assessment of Fish and Fisheries of the Ganga River System for Developing Suitable Conservation and Restoration Plan”
MID TERM REPORT ICAR - CENTRAL INLAND FISHERIES RESEARCH INSTITUTE ‟Assessment of fish and fisheries of the Ganga river system for developing suitable conservation and restoration plan” (Sanctioned under National Mission on Clean Ganga, vide NGRBA Order NO.T-17 /2014 15/526/NMCG-Fish and Fisheries Dated 13/07/2015) PROJECT TEAM Principal Investigator Dr. Basanta Kumar Das Co-Principal Investigators Dr. Ranjan Kumar Manna Dr. Rama Shanker Srivastava Dr. Dharam Nath Jha Mr. Raju Baitha Mr. Himanshu Sekhar Swain Mr. Mitesh Hiradas Ramteke Ms. Thangjaum Nirupada Chanu Mr. Jeetendra Kumar Mr. Shayamal Chandra Sukla Das Ms. Canciyal Johnson Ms. Tanushree Bera Dr. Monika Gupta Research Associate Mr. Hari Om Verma Senior Research Fellow Mr. Archisman Ray Ms. Supriti Bayen Mr. Sandeep Kumar Mishra Mr. Sushil Kumar Verma Technical officer Mr. Lokenath Chakraborty Mr. Subhendu Mandal Mr. Samir Kumar Paul Mr. Ashis Roychowdhury Young Professional Ms. Manisha Bhor Mr. Subhadeep Das Gupta Mr. Nitish Kumar Tiwari Ms. Trupti Rani Mohanty Ms. Jahanara Seikh Submitted to: National Mission for Clean Ganga (Ministry of Water Resources, River Development and Ganga Rejuvenation) 1st Floor, Major Dhyan Chand National Stadium, India Gate, New Delhi- 110002 Date of submission: 14th February, 2019 2 LIST OF TABLES Table 1. State and distance wise distribution of sampling stations in river Ganga Table 2. Review on number of fish species identified from Ganga River Table 3. Habitat wise family distribution along the stretch of river Ganga Table 4. Checklist of fishes identified from Ganga basin under the CIFRI- NMCG project. Table 4 (a). Threat status of fishes recorded from river Ganga. -

Indian Ocean, Eastern
526 Fish, crustaceans, molluscs, etc Capture production by species items Indian Ocean, Eastern C-57 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Océan Indien, est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Océano Índico, oriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2012 2013 2014 2015 2016 2017 2018 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 113 86 94 66 55 93 80 Chacunda gizzard shad Anodontostoma chacunda 24 8 251 8 320 11 077 9 012 10 761 10 504 11 055 Hilsa shad Tenualosa ilisha 24 246 216 264 144 261 826 268 115 328 095 328 991 300 419 Toli shad Tenualosa toli 24 267 161 212 80 203 95 219 Indian pellona Pellona ditchela 24 9 016 10 883 12 501 15 341 13 319 13 797 15 637 Diadromous clupeoids nei Clupeoidei 24 45 208 30 170 131 42 25 Barramundi(=Giant seaperch) Lates calcarifer 25 11 027 11 085 10 512 11 149 9 531 14 090 11 128 Tonguefishes Cynoglossidae 31 3 473 2 912 2 191 2 436 2 923 3 647 3 782 Indian halibut Psettodes erumei 31 11 312 12 368 10 606 7 709 8 533 6 215 9 141 Flatfishes nei Pleuronectiformes 31 14 584 18 415 19 943 15 278 15 770 14 752 17 177 Unicorn cod Bregmaceros mcclellandi 32 2 680 5 132 300 190 80 241 62 Blue grenadier Macruronus novaezelandiae 32 4 080 4 019 4 002 1 379 1 727 1 425 1 537 Grenadiers, rattails nei Macrouridae 32 1 - - - - - - Gadiformes nei Gadiformes 32 11 10 11 13 16 9 7 Indo-Pacific tarpon Megalops cyprinoides 33 32 19 27 33 -

Of 14 CLASSIFICATION SCIENTIFIC NAME ENGLISH NAME LOCAL NAME R-9 R-10 R-11 R-12 Prices in Peso Dasyatis Spp
REGIONAL WEEKLY PREVAILING RETAIL (PER KILO) PRICE MONITORING REPORT FOR THE MONTH OF MAY 1-31, 2017 ALL REGIONS CLASSIFICATION SCIENTIFIC NAME ENGLISH NAME LOCAL NAME R-9 R-10 R-11 R-12 Prices in Peso A. FINFISHES A.1 Marine Acanthocybium solandri Wahoo Tanige/Tanguige 140-180 Acanthurus olivaceus Surgeonfish Indangan 140-220 Alectis ciliaris African Pampano trakito/talakitok 160-250 Alectis ciliaris African Pampano BIG Alectis ciliaris African Pampano MEDIUM Alepes melanoptera Round scad Galonggong 100-120 Amblygaster sirm Spotted sardinella Tamban/hawol-hawol Atule mate Yellow tail scad Bagudlong/Budburon/kalapato/Salay-salay 90.00 Atule mate Yellowtail scad BIG Atule mate Yellowtail scad MEDIUM Auxis rochei Bullet Tuna Aloy/Tulingan/perit/bodboron 100.00 120.00 70-140 Auxis rochei Bullet tuna BIG Auxis rochei Bullet tuna MEDIUM Auxis rochei Bullet tuna SMALL Auxis spp Mackarel Pirit/ Bodboron 100-120 Auxis thazard Frigate tuna Tulingan/turingan/pidlayan/Tangigi/mangko 120.00 60-120 Auxis thazard Frigate Tuna BIG Auxis thazard Frigate tuna MEDIUM Balistapus undulatus Orange-lined trigger fish Pugot 60-100 Balistoides viridescens Titan triggerfish Pakol 100.00 Caesio caerulaurea Blue & Gold Fusilier BIG Caesio caerulaurea Blue & Gold Fusilier MEDIUM Caesio caerulaurea Blue & Gold Fusilier SMALL Caesio cuning Redbelly yellowtail fusilier Dalagang Bukid 170-180 180-220 Caesio lunaris Lunar fusilier Dalagang bukid 200.00 Caesio spp. Splitted caesio Dalagang Bukid 100-250 Canthidermis maculata Rough triggerfish Pakol/pugot 60-70 Caranx georgianus Trevally Talakitok 200-320 Caranx ignobilis Giant trevally Maliputo / Talakitok/isdaputih/mangsah 160.00 Caranx ignobilis Giant trevally BIG Caranx ignobilis Giant trevally MEDIUM Caranx spp. -

And Their Fisheries In
SMALL PELAGIC RESOURCES AND THEIR FISHERIES IN THE ASIA-PACIFIC REGION ASIA-PACIFIC FISHERY COMMISSION FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS REGIONAL OFFICE FOR ASIA AND THE PACIFIC RAP Publication 1997/31 SMALL PELAGIC RESOURCES AND THEIR FISHERIES IN THE ASIA-PACIFIC REGION Proceedings of the First Session of the APFIC Worldng Party on Marine Fisheries Bangkok, Thailand, 13-16 May 1997 Asia-Pacific Fishery Commission Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific Bangkok, Thailand, 1997 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of itsauthorities, or concerning the delinutation of its frontiers or boundaries. Opinions expressed in this publication are those of the author(s) alone and do not imply any opinion whatsoever on the part of FAO and APFIC. NOTICE OF COPYRIGHT The copyright in this publication is vested in the Food and Agriculture Organization of the United Nations. This publication may not be reproduced, in whole or in part, by any method or process, without written permission from the copyright holder. Applications for such permission with a statement of the purpose and extent of the reproduction desired should be made through and addressed to the Senior Fishery Officer, FAO Regional Office for Asia and the Pacific, Maliwan Mansion, Phra Athit Road, Bangkok 10200, Thailand. ii PREPARATION OF THIS DOCUMENT This Proceedings consists of the Conclusion and Recommendations of and the papers presented at the First Session of the APFIC Working Party on Marine Fisheries held in Bangkok, Thailand, from 13 to 16 May 1997.