PNEUMOVAX 23 Safely and Effectiv Ely
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

362.Full.Pdf
AMERICAN ACADEMY OF PEDIATRICS Committee on Infectious Diseases Policy Statement: Recommendations for the Prevention of Pneumococcal Infections, Including the Use of Pneumococcal Conjugate Vaccine (Prevnar), Pneumococcal Polysaccharide Vaccine, and Antibiotic Prophylaxis ABSTRACT. Heptavalent pneumococcal conjugate vac- lular pertussis; HbOC, Haemophilus influenzae type b conjugate cine (PCV7) is recommended for universal use in chil- vaccine; HIV, human immunodeficiency virus; AOM, acute otitis dren 23 months and younger, to be given concurrently media. with other recommended childhood vaccines at 2, 4, 6, and 12 to 15 months of age. For children 7 to 23 months he purpose of this report is to provide recom- old who have not received previous doses of PCV7, ad- ministration of a reduced number of doses is recom- mendations for use of the heptavalent pneumo- mended. Two doses of PCV7 are recommended for chil- Tcoccal conjugate vaccine (PCV7), Prevnar (Led- dren 24 to 59 months old at high risk of invasive erle Laboratories, Pearl River, NY; Wyeth-Ayerst pneumococcal infection—including children with func- Pharmaceuticals, Marietta, PA), and 23-valent pneumo- tional, anatomic, or congenital asplenia; infection with coccal polysaccharide (23PS) vaccines. In addition, rec- human immunodeficiency virus; and other predisposing ommendations for the continuing use of antibiotic pro- conditions—who have not been immunized previously phylaxis in children with sickle cell disease (SCD) and with PCV7. Recommendations have been made for use of asplenia will be given, and the use of antibiotics and 23-valent pneumococcal polysaccharide (23PS) vaccine in vaccines in children who attend out-of-home care will high-risk children to expand serotype coverage. -

Pneumococcal Vaccine Timing for Adults Make Sure Your Patients Are up to Date with Pneumococcal Vaccination
Pneumococcal Vaccine Timing for Adults Make sure your patients are up to date with pneumococcal vaccination. When both are If either vaccine is PCV13 and PPSV23 Two pneumococcal vaccines are recommended for adults: indicated, PCV13 inadvertently given should not be 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13®) should be given earlier than the administered during the ® before PPSV23 recommended window, 23-valent pneumococcal polysaccharide vaccine (PPSV23, Pneumovax 23) same office visit. whenever possible. do not repeat the dose. One dose of PCV13 is recommended for adults: One dose of PPSV23 is recommended for adults: 19 years or older with certain medical conditions and who have not 65 years or older, regardless of previous history of vaccination with previously received PCV13. See Table 1 for specific guidance. pneumococcal vaccines. Adults 65 years or older can discuss and decide, with their clinician, – Once a dose of PPSV23 is given at age 65 years or older, no to receive PCV13 if they have not previously received a dose (shared additional doses of PPSV23 should be administered. clinical decision-making). 19 through 64 years with certain medical conditions. – A second dose may be indicated depending on the medical condition. See Table 1 for specific guidance. Adults 65 years or older without an immunocompromising condition, CSF* leak, or cochlear implant For those who have not received any pneumococcal For those who have previously received 1 dose of vaccines, or those with unknown vaccination history PPSV23 at ≥ 65 years and no doses of PCV13 If patient and provider decide PCV13 is not to be given: If patient and provider decide PCV13 is not to be given: Administer 1 dose of PPSV23. -

Adults with DIABETES Are Among Those Who Need Pneumococcal
Patients with diabetes are at an increased risk for complications from pneumococcal disease. Diabetes may be a unique risk factor for increased incidence of sepsis associated with pneumococcal infection. One of the reasons people with diabetes are at greater risk for pneumococcal disease is that they may have abnormalities in immune function that affect their reaction to infection. Pneumococcal disease causes serious illnesses like pneumonia, meningitis, and sepsis. Pneumococcal disease is serious and deadly. In the US, pneumococcal pneumonia, meningitis, and sepsis kill tens of thousands each year. Pneumococcal disease survivors may suffer hearing loss, seizures, blindness, or paralysis. Pneumococcal vaccination is recommended for all adults with diabetes. Adults with DIABETES Are Among Those Who Need Pneumococcal Vaccination There are two types of pneumococcal vaccine recommended for US adults: a pneumococcal conjugate vaccine (PCV13) and a pneumococcal polysaccharide vaccine (PPSV23). Adults 65 and older, and adults age 19 to 64 years with any of the following need to receive both vaccines: immunocompromising conditions or treatments (e.g., HIV/AIDS, leukemia, lymphoma, Hodgkin disease, radiation therapy); a damaged or missing spleen; cochlear implants; or cerebrospinal fluid leaks. Other adults for whom pneumococcal vaccination is recommended only need PPSV23, but may need more than one dose and will need PCV13 when they, too, reach age 65. Please refer to the Adult Pneumococcal Vaccination Guide or visit cdc.gov/vaccines/vpd-vac/pneumo/ for details on timing of vaccine doses. For more information and resources to educate patients about pneumococcal disease, visit adultvaccination.org/professional-resources/pneumo This initiative is supported by unrestricted educational grants from Merck & Co., Inc. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -

Global Immunization News 25 February 2011
Global Immunization News 25 February 2011 World Health Organization Global Immunization News Inside this issue: Technical Information Meeting of the Global Advisory 2 Committee on Vaccine Safety Polio eradication mourns loss of 2 NEW VACCINES, NEW true polio champion OPPORTUNITIES Announcements from The SIVAC 3 25/02/2011 from Hayatee Hasan, WHO/HQ Initiative In the past two months, four countries ― WHO position on pandemic influ- 3 Guyana, Kenya, Sierra Leone and Yemen ― enza vaccination following reports of narcolepsy subsequent to use of have introduced the pneumococcal conjugate Pandemrix vaccine. They represent the first of a series of countries introducing the vaccine in 2011. PHOTO Cold Chain and Logistics Taskforce 3 WHO concludes that quality issues 4 These introductions represent a major relating to Quinvaxem production milestone - the gap between access to new have been resolved vaccines between developed and developing AFRICA 4-6 countries is shortening; it is extraordinary to The launching of the Pneumococcal see a new vaccine launched in a developing conjugate vaccine (PCV-13) in Si- erra Leone country within one to two years of its introduction in the Americas and Europe, Kenya launches ten-valent pneumo- while in the past, it has taken several years coccal conjugate vaccine (PCV10) Vaccine carrier containing pneumococcal vaccine in (averaging 15 years) between the Kenya Meetings held in afro central introduction of new vaccines in developed and developing countries. AMERICAS 6-8 Peru introduces nationwide HPV vaccination; Argentina announces For more information regarding the launches in these countries, please see the articles on HPV vaccine introduction Kenya, Sierra Leone and Yemen. -

Vaccines-Pneumococcal- Influenza- Shingles Q and A
www.RxFiles.ca - July 2019 RxFiles Q&A Summary A Crawley BSP; J Bareham BSP VACCINES: Pneumococcal, Influenza, & Shingles Immunization Guidelines and Saskatchewan Health Coverage Considerations Vaccines prevent morbidity and mortality to various degrees. Many vaccines are publicly funded, especially if potentially life-saving. When guideline recommendations & public coverage differ, clinicians/patients must weigh the evidence for benefit versus the out-of-pocket patient cost. Note: vaccine costs listed in this document do not include markup, dispensing fees, or administration fees, which can vary depending on who is administering the vaccine. 1. Who may benefit from a PNEUMOCOCCAL vaccine in Saskatchewan? Available vaccines include the PNEUMOVAX 23-valent vaccine ($24) and the PREVNAR 13-valent vaccine ($103). Covered in Sask:1 Evidence for benefit Clinical Controversies 1 dose of PNEUMOVAX for anyone A single pneumococcal vaccination Immunization guidelinesNACI suggest that if ≥65 years old. appears to reduce the risk of PNEUMOVAX was given before the age of 65, a booster 1 dose of PNEUMOVAX for anyone community-acquired pneumonia by dose should be given 5 years later to all patients with specific medical conditions 30% (NNT = 55) and the risk of a COPD regardless of risk factors.18 This recommendation is (e.g. diabetes, COPD, others*). exacerbation by 40% (NNT = 8).2,19,20 based on the tendency for older adults to have a 2 doses of PNEUMOVAX spaced 5+ Benefits are consistent regardless of weakened immune system. years apart for anyone with which vaccine formulation is used.2-4 Immunization guidelines suggest that the theoretical specific high risk medical No trials have yet assessed the efficacy greater potency of PREVNAR over PNEUMOVAX may conditions (e.g. -

Pneumococcal Vaccine Called PCV7, Or to Any Vaccine Spread from Person to Person Through Close Contact
VACCINE INFORMATION STATEMENT Pneumococcal Conjugate Vaccine (PCV13) Many Vaccine Information Statements are available in Spanish and other languages. See www.immunize.org/vis Hojas de informacion sobre vacunas est&n What You Need to Know disponibles en espafiol y en muclios otros idiomas, Visite www.immunize.org/vis 1 Why get vaccinated? ] Some people should not get this vaccine Vaccination can protect both children and adults from pneumococcal disease. Anyone who has ever had a life-threatening allergic reaction to a dose of this vaccine, to an earlier Pneumococcal disease is caused by bacteria that can pneumococcal vaccine called PCV7, or to any vaccine spread from person to person through close contact. It containing diphtheria toxoid (for example, DTaP), can cause ear infections, and it can also lead to more should not get PC VI3. serious infections of the: • Lungs (pneumonia),' Anyone with a severe allergy to any component of • Blood (bacteremia), and PC VI3 should not get the vaccine. Tell your doctor if the • Covering of the brain and spinal cord (meningitis). person being vaccinated has any severe allergies. Pneumococcal pneumonia is most common among If the person scheduled for vaccination is not feeling adults. Pneumococcal meningitis can cause deafness and well, your healthcare provider might decide to brain damage, and it kills about 1 child in 10 who get it. reschedule the shot on another day. Anyone can get pneumococcal disease, but children under 2 years of age and adults 65 years and older, Risks of a vaccine reaction people with certain medical conditions, and cigarette With any medicine, including vaccines, there is a chance smokers are at the highest risk. -

Adult Pneumococcal Vaccination Recommendations
Adult Pneumococcal Vaccination Recommendations Background1,2 Streptococcus pneumoniae is a leading cause of invasive disease, including bacteremia, meningitis, and pneumonia in the United States. It is estimated to be responsible for 4 million episodes of illness, 445,000 hospitalizations, and 22,000 deaths per year. Incidence of invasive disease ranges from 3.8 cases per 100,000 persons among adults aged 18-34 years to 36.4 cases per 100,000 persons among adults aged ≥ 65 years. Patients with high-risk immunocompromising medical conditions, such as hematologic malignancies and human immunodeficiency virus, have up to a 20-fold increased risk for invasive pneumococcal disease. The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) currently recommends pneumococcal for all adults aged ≥ 65 years, as well as adults aged 19-64 years with immumocompromising conditions. Two pneumococcal vaccines are available, 23-valent pneumococcal polysaccharide vaccine (PPSV23) and 13-valent polysaccharide vaccine (PCV13). The recommendations below outline the preferred formulations and vaccination schedules according to the ACIP guidelines. Pneumococcal Vaccination for Adults Aged ≥ 65 Years3,4 In 2010, ACIP recommended that all adults should be vaccinated with PPSV23 at age 65 years. In 2014, these recommendations were updated based on results from a randomized, placebo-controlled trial showing efficacy of PCV13 in preventing pneumonia in 85,000 adults aged ≥ 65 years. ACIP now recommends that both PCV13 and PPSV23 should be routinely administered to all adults aged ≥ 65 years. In addition, ACIP recently revised the recommended intervals for sequential use of PCV13 and PPSV23 in this patient population. -

Pneumococcal Vaccination Recommendations
D E I S L A O N H Pneumococcal Vaccination D R D H E T Recommendations P L A 1-4 COLLEGE OF R A Adults ≥19 Years PHARMACY T E M H DRUG INFORMATION F E N T O (Including updated recommendations for the use of PCV13 in Adults) SERVICES 401-874-9188 Healthy Adults ≥ 65 Pneumococcal Vaccination Previously vaccinated with Previously vaccinated with Naive or Unknown History PPSV23 at age ≥65 PPSV23 before age 65 ≥ 1 year after PPSV23 GIVE: PCV13 ≥ 1 year after PPSV23 GIVE: PCV13 if not previously given Wait ≥ 1 year* Wait ≥ 1 year* (and ≥ 5 years after PPSV23) GIVE: PPSV23† GIVE: PCV13 if not previously given GIVE: PPSV23† ADULTS ≥ 19 with UNDERLYING MEDICAL CONDITIONS (see chart on back) OR who SMOKE or live in a NURSING HOME Pneumococcal Vaccination Previously vaccinated with one Naive or Unknown History dose PPSV23 Vaccination is NOT indicated for healthy persons GIVE: PPSV23 19 - 64 years of age While PCV13 is FDA-approved for persons > 50 years, the Advisory At Age ≥65 At Age ≥65 Committee on Immune Practices GIVE: PCV13 ≥ 1 year after PPSV23 GIVE: PCV13 ≥ 1year after PPSV23 does not provide guidance for use THEN: PPSV23† ≥ 1 year* after THEN: PPSV23† ≥ 1 year* after in this population. PCV13 and ≥ 5 years after PPSV23 PCV13 and ≥ 5 years after PPSV23 ADULTS ≥ 19 with IMMUNE COMPROMISING CONDITIONS (see chart on back), OR ASPLENIA (including sickle cell anemia), CEREBROSPINAL FLUID LEAK, or COCHLEAR IMPLANT Pneumococcal Vaccination Previously vaccinated with one Previously vaccinated with Naive or Unknown History dose PPSV23 two doses of -

Immunizers Guide to Flu and PPV Vaccinations
2009-2010 Immunizers’ Question & Answer Guide to Medicare Coverage of Seasonal Influenza and Pneumococcal Vaccinations Steps to Promoting Wellness Adult Immunizations The issues involved in Medicare billing and administration can be complex and may vary state to state. For this reason, we recommend that you contact your local fiscal intermediary/AB MAC, carrier/AB MAC (Part B), or the Centers for Medicare & Medicaid Services’ Regional Office for more detailed information. Centers for Medicare & Medicaid Services Medicare Influenza and Pneumococcal Vaccination Benefits 2009-2010 Immunizer’s Q&A Guide to Medicare Coverage9/23/2009 Table of Contents A. Introduction ........................................................................................................................................... 4 Purpose ................................................................................................................................................ 4 2009-2010 Update on H1N1 ............................................................................................................... 4 Background of Medicare Pneumococcal and Seasonal Influenza Vaccination Benefits .................... 5 ACIP Guidelines .................................................................................................................................. 5 Summary of ACIP Guidelines ............................................................................................................. 6 Who Should Not Be Vaccinated ...................................................................................................... -

Immunization Recommendations for College Students
OCTOBER 2018 ACHA Guidelines Immunization Recommendations for College Students mmunizations offer safe and effective protection from vaccine-preventable diseases and outbreaks. The United States is experi- encing re-emergence of these diseases, in part due to factors such as un-immunized and under-immunized persons and global travel. I The American College Health Association (ACHA) strongly supports the use of vaccines to protect the health of our individual students and our campus communities. In recognition of the vital role that vaccine coverage plays in community immunity (herd immunity), ACHA discourages use of nonmedical exemptions to required vaccines. This guidance is provided to facilitate implementation of a comprehensive institutional immunization policy. Best practices for institu- tions of higher education include the following Immunization Recommendations for College Students (IRCS), encouraging students who request nonmedical exemptions to required vaccines to be counseled by a health service clinician, and considering exclusion of un- immunized students from school during outbreaks of vaccine-preventable diseases. Institutions may also be subject to additional requirements for pre-matriculation vaccinations and the granting of exemptions by state law. The ACHA Vaccine-Preventable Diseases Advisory Committee updates this document in accordance with changing public health rec- ommendations. These guidelines follow Advisory Committee on Immunization Practices (ACIP) recommendations published by the U.S. Centers for Disease -
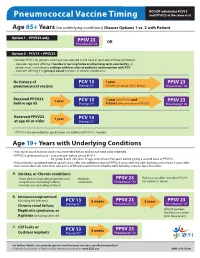
Pneumococcal Vaccine Timing and PPSV23 at the Same Visit
DO NOT administer PCV13 Pneumococcal Vaccine Timing and PPSV23 at the same visit. Age 65+ Years (no underlying conditions) Discuss Options 1 vs. 2 with Patient Option 1 – PPSV23 only PPSV 23 Pneumovax® 23 OR Option 2 – PCV13 + PPSV23 Consider PCV13 for persons who have not received it and have at least one of these conditions: • Consider regularly oering if resides in nursing home or other long-term care facility, or resides in or is traveling to settings with low rates of pediatric immunization with PCV. • Consider oering if in group A below (smoker, or chronic conditions). No history of PCV 13 1 year PPSV 23 pneumococcal vaccine Prevnar13® (8 weeks for groups B & C below) Pneumovax® 23 Received PPSV23 1 year PCV 13 1 year after PCV13 and PPSV 23 before age 65 Prevnar13® 5 years after prior dose of PPSV23 Pneumovax® 23 Received PPSV23 1 year PCV 13 at age 65 or older Prevnar13® • If PCV13 was given before age 65 years, no additional PCV13 is needed. Age 19+ Years with Underlying Conditions • Prior doses count towards doses recommended below and do not need to be repeated. • If PPSV23 given previously – wait one year before giving PCV13 – for group B and <65 years of age, wait at least ve years before giving a second dose of PPSV23. • If doses below completed before age 65 years, oer one additional dose of PPSV23 on or after the 65th birthday and at least 5 years after most recent dose. No more than two doses of PPSV23 recommended before 65th birthday and one dose thereafter.