Groundwater Dynamics, Paleoclimate and Noble Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whither Sustainability? Governance and Regional Integration in the Glatt Valley1
Whither sustainability? Governance and regional integration in the Glatt Valley1 Constance Carr, Ph.D. & Evan McDonough, Ph.D. Candidate Institute of Geography and Spatial Planning University of Luxembourg Luxembourg presented at Regional Studies Association Research Network, “How to govern fundamental Sustainability Transition processes?” 11 July 2014, St. Gallen, Switzerland Abstract This paper problematises the concept and practice of integrative planning – one of the central tenants of sustainability. We contend that, in practice, planning for the broader goal of spatial integration has the effect of producing a fundamentally paradoxical and contradictory social space, a form of urbanisation (or suburbanisation) that reinforces some of the problems which sustainability seeks to address. Drawing on an empirical base of observations of transport integration initiatives in the region of the Glatt Valley, and interview work in the field, this paper examines how integrative spatial planning strategies sanction further fragmentation. Observed in the Glatt Valley were attempts to consolidate infrastructure towards optimising capital accumulation along particular axes of flows. Housing, transport, and economic development were three key areas that required integration. The apparent integration of the region, however, is contrasted against a fragmented field of governance and an ambiguous set of winners and losers. The research confirms that integrative strategies can entrench and exacerbate existing tendencies of fragmented governance, and in fact, generate new rounds of fragmentation with respect to land use and social worlds. Introduction There is not much dispute about the broad definition of sustainable development – that it spans economic, social, and environmental issues, and that while these can be conceived as pillars, concentric circles or a Venn diagram (Rydin 2010), the main goal towards achieving sustainability is the integration these three dimensions. -

UNITED NATION D NS St on Po Secon Ockholm N Persiste Ollutants
UNITED NATIONS SC UNEP/POPS/COP.8/INF/38 Distr.: General Stockholm Convention 23 January 2017 on Persistent Organic English only Pollutants Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants Eighth meeting Geneva, 24 April–5 May 2017 Item 5 (i) of the provisional agenda* Matters related to the implementation of the Convention: effectiveness evaluation Second global monitoring report Note by the Secretariat As referred to in the note by the Secretariat on the global monitoring plan for effectiveness evaluation (UNEP/POPS/COP.8/21), the second global monitoring report prepared by the global coordination group is set out in the annex to the present note. The executive summary of the report, including the conclussions and recommendations of the global coordination group, is also reproduced in the six official languages of the United Nations in document UNEP/POPS/COP.8/21/Add.1. The present note, including its annex, has not been formally edited. * UNEP/POPS/COP.8/1. 080217 UNEP/POPS/COP.8/INF/38 Annex Second global monitoring report 2 UNEP/POPS/COP.8/INF/38 TABLE OF CONTENTS ACKNOWLEDGEMENTS ............................................................................................................ 5 PREFACE ....................................................................................................................................... 6 ABBREVIATIONS AND ACRONYMS ....................................................................................... 7 GLOSSARY OF TERMS ............................................................................................................ -

Inaugural – Dissertation
INAUGURAL – DISSERTATION Zur Erlangung der Doktorwürde der Naturwissenschaftlich-Mathematischen Gesamtfakultät der Ruprecht-Karls-Universität Heidelberg Analysenmethoden für Antibiotika und perfluorierte Tenside in wässrigen Matrizes mittels LC-MS/MS nach SPE-Anreicherung - Methodenentwicklung, Methodenvalidierung, Datenerhebung vorgelegt von Lebensmittelchemiker Dirk Skutlarek aus Osnabrück Bonn 2008 INAUGURAL – DISSERTATION Zur Erlangung der Doktorwürde der Naturwissenschaftlich-Mathematischen Gesamtfakultät der Ruprecht-Karls-Universität Heidelberg vorgelegt von Lebensmittelchemiker Dirk Skutlarek aus Osnabrück Bonn 2008 Tag der mündlichen Prüfung: 21. November 2008 2 Analysenmethoden für Antibiotika und perfluorierte Tenside in wässrigen Matrizes mittels LC-MS/MS nach SPE-Anreicherung - Methodenentwicklung, Methodenvalidierung, Datenerhebung Gutachter: Prof. Dr. Heinz Friedrich Schöler Institut für Umwelt-Geochemie Universität Heidelberg Prof. Dr. Thomas Braunbeck Institut für Zoologie Universität Heidelberg Priv.-Doz. Dr. Thomas Heberer Lebensmittelinstitut Oldenburg Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit 3 4 meiner Familie 5 Inhaltsverzeichnis Abstract 10 Zusammenfassung 11 1. Analysenmethoden für Antibiotika und perfluorierte Tenside in wässrigen Matrizes mittels LC-MS/MS nach SPE-Anreicherung - Methodenentwicklung, Methodenvalidierung, Datenerhebung 14 1.1. Grundlagen 14 1.1.1. Allgemeines 14 1.1.2. Arzneimittel – Antibiotika 15 1.1.2.1. Allgemeines zur Problematik von Arzneimitteln / Antibiotika in der Umwelt 15 1.1.2.2. Antibiotika-Verbrauchsmengen in Deutschland 16 1.1.2.3. Entwicklungsgeschichte der Antibiotika 19 1.1.2.4. Einteilung der Antibiotika anhand ihrer Wirkmechanismen und Wirkungstypen 20 1.1.2.5. Resistenzbildung von Mikroorganismen gegenüber Antiinfektiva 22 1.1.2.6. Penicilline 22 1.1.2.7. Makrolide 23 1.1.2.8. Glykopeptide: Vancomycin 24 1.1.2.9. Fluorchinolone (Gyrasehemmer) 24 1.1.2.10. Sulfonamide 25 1.1.2.11. -

A ~180,000 Years Sedimentation History of a Perialpine Overdeepened Glacial Trough (Wehntal, N-Switzerland)
Swiss J Geosci (2010) 103:345–361 DOI 10.1007/s00015-010-0041-1 A ~180,000 years sedimentation history of a perialpine overdeepened glacial trough (Wehntal, N-Switzerland) Flavio S. Anselmetti • Ruth Drescher-Schneider • Heinz Furrer • Hans Rudolf Graf • Sally E. Lowick • Frank Preusser • Marc A. Riedi Received: 29 June 2009 / Accepted: 3 March 2010 / Published online: 25 November 2010 Ó Swiss Geological Society 2010 Abstract A 30 m-deep drill core from a glacially over- indicating a warming of climate. These sediments are deepened trough in Northern Switzerland recovered a overlain by a peat deposit characterised by pollen assem- *180 ka old sedimentary succession that provides new blages typical of the late Eemian (MIS 5e). An abrupt insights into the timing and nature of erosion–sedimenta- transition following this interglacial encompasses a likely tion processes in the Swiss lowlands. The luminescence- hiatus and probably marks a sudden lowering of the water dated stratigraphic succession starts at the bottom of the level. The peat unit is overlain by deposits of a cold core with laminated carbonate-rich lake sediments reflect- unproductive lake dated to late MIS 5 and MIS 4, which do ing deposition in a proglacial lake between *180 and not show any direct influence from glaciers. An upper peat 130 ka ago (Marine Isotope Stage MIS 6). Anomalies in unit, the so-called «Mammoth peat», previously encoun- geotechnical properties and the occurrence of deformation tered in construction pits, interrupts this cold lacustrine structures suggest temporary ice contact around 140 ka. phase and marks more temperate climatic conditions Up-core, organic content increases in the lake deposits between 60 and 45 ka (MIS 3). -

Antibiotic Resistance Gone Wild
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1617 Antibiotic resistance gone wild A One Health perspective on carriage, selection and transmission of Extended-Spectrum Cephalosporinase- and Carbapenemase-producing Enterobacteriaceae CLARA ATTERBY ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 ISBN 978-91-513-0817-3 UPPSALA urn:nbn:se:uu:diva-397218 2019 Dissertation presented at Uppsala University to be publicly examined in Tripple room, Navet ground floor, BMC, Husargatan 3, Uppsala, Friday, 24 January 2020 at 09:00 for the degree of Doctor of Philosophy (Faculty of Medicine). The examination will be conducted in English. Faculty examiner: Professor Nicola Williams (Institute of Infection and Global Health in Liverpool, United Kingdom). Abstract Atterby, C. 2019. Antibiotic resistance gone wild. A One Health perspective on carriage, selection and transmission of Extended-Spectrum Cephalosporinase- and Carbapenemase- producing Enterobacteriaceae. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1617. 79 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0817-3. Antibiotics have saved millions of lives since they came into clinical use during the Second World War in the 1940s. Today, our effective use of antibiotics is under great threat due to emerging antibiotic resistance in bacteria. This thesis addresses the problems of antibiotic resistance from a ”One Health” perspective. The focus is on antibiotic resistant Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae) in the environment and wildlife, and also considering the situation in healthy humans and livestock. In Paper I-III, high occurrence of Extended-Spectrum Beta-Lactamase (ESBL) -producing E. coli and/or K. -

Biogeochemical Behaviour of Alkvlphenol Pol Vethoxvlates in the Aquatic Environment
BIOGEOCHEMICAL BEHAVIOUR OF ALKVLPHENOL POL VETHOXVLATES IN THE AQUATIC ENVIRONMENT A dissertation submitted to the UNIVERSITY OF ZAGREB INSTITUTE "RUDJER BO~KOVIC" for the degree of DOCTOR OF NATURAL SCIENCES presented by MARIJAN AHEL dipl. biotechnol., University of Zagreb M.Sc. Marine Sciences, University of Zagreb born on 3 October 1951 citizen of Zagreb, Croatia, Yugoslavia Accepted on recommendation of Dr. Walter Giger Dr. Bo~ena Cosovic Dr. Nikola Bl~evic Dr. Sonja lskric Dr. Leo Klasinc Zagreb 1987 The following parts of this dissertation have been published: Section 3.1. Analytical Chemistry 1985, 57, 1577-1583. Analytical Chemistry 1985, 57, 2584-2590. Environmental Science & Technology 1987, 21, 697-703. Section 3.3. Vom Wasser 1986, 67, 69-81. Water Science & Technology 1987, 19, 449-460. Gas-Wasser-Abwasser 1987, 67, 111-122. 'Io my sons I van, 'Ivrt{\g, Ja{\gv, and Juraj ACKNOWLEDGEMENTS I am especially greatful to Dr. Walter Giger for his whole hearted support during this study. I have found in him an inspired teacher and a dear friend. I owe my special thanks to Professor Werner Stumm, Director of the Swiss Federal Institute for Water Resources and Water Pollution Control, who made it possible for me to work in this renowned Institution. I am also indebted to Dr. Bozena Cosovic for the encouragement and under- standing and for useful advice while writing this thesis. This dissertation would not have been possible without the support of many collegues and friends from the Chemistry Department of EAWAG and from the Center for Marine Research Zagreb of the Institute "Rudjer Boskovic" to whom I am indebted. -

NEALTINE and Meat De(Lt. Chi^Roll »69 Pot Roast 3 5 5 9 6 9 '
■t W i ' r ■' V . i >'/: •/ ¥s..I' i ^OBTWmY-FOUB THURSDAY, OCTOBER 11,1966 Avaragir Daily Net PreM Run Tha Waatkar. F m - the W m V Kad#d ^attrliP0t(r lEtt^nins ISmtUt Oot a. itM ' - ■ It .e V. 8. Wi FiUr. Helen Da'vldirdn Lodge, No. M, Miss Zlisabeth Bonturl's first Miaa Jean Macfarlan, Garden u oO tm U m g Daughters .of • Scolla,'wtir hold 'a grade .won the-attendance banner Apta., Had aa weekend guests Mrs. 1 2 ,2 8 3 tonight m M tetn tia y . A bou t Tow n meeting at the Masonic Temple et the Nathan Hale PTA meeting Aghea DeRemer, her, niece and M tm h» mt tk. Avdit tonight. I.OTr S«-«a^ tomorrow night at 7:46., ' this week Instead of the fifth nephew, Mr. and Mrs. Edward Bu n m . f OUcalatiMi •a-86. ' "lly faltlj In My Job,” the lec- grade, as previously reported. ^ Hin, and Masters James and ' Pinehurst at PE^Mam.s.O aiid Friday Nights Till 9d)0 M ancheMter-—^A City o f Village Charm oa d ln • ••tie* of dlscuMion*. will Brendan Shea, son of Mr. and George Hln, all of Paterson, N. J. be belli tonight *t « o’clock in the Mrs. William J. Shea. Boulder Rd., The American . Legion ., Auxil yiMlenUon room »t Center Con- has recently been pledged to Alpha iary will meet .Monday nlghj, at 8 The legular weekly meeting of i T0fc^t.xxvf, NO. 11 , / (TWENTY PAGES) MANCHESTER, CONN., FRIDAY, OCTOBER^, 1956 (ClnNlfled Advertlilng on Pago 18) PRICE. -

Quaternary Glaciation History of Northern Switzerland
Quaternary Science Journal GEOzOn SCiEnCE MEDiA Volume 60 / number 2–3 / 2011 / 282–305 / DOi 10.3285/eg.60.2-3.06 iSSn 0424-7116 E&G www.quaternary-science.net Quaternary glaciation history of northern switzerland Frank Preusser, Hans Rudolf Graf, Oskar keller, Edgar krayss, Christian Schlüchter Abstract: A revised glaciation history of the northern foreland of the Swiss Alps is presented by summarising field evidence and chronologi- cal data for different key sites and regions. The oldest Quaternary sediments of Switzerland are multiphase gravels intercalated by till and overbank deposits (‘Deckenschotter’). Important differences in the base level within the gravel deposits allows the distin- guishing of two complex units (‘Höhere Deckenschotter’, ‘Tiefere Deckenschotter’), separated by a period of substantial incision. Mammal remains place the older unit (‘Höhere Deckenschotter’) into zone MN 17 (2.6–1.8 Ma). Each of the complexes contains evidence for at least two, but probably up-to four, individual glaciations. In summary, up-to eight Early Pleistocene glaciations of the Swiss alpine foreland are proposed. The Early Pleistocene ‘Deckenschotter’ are separated from Middle Pleistocene deposition by a time of important erosion, likely related to tectonic movements and/or re-direction of the Alpine Rhine (Middle Pleistocene Reorganisation – MPR). The Middle-Late Pleistocene comprises four or five glaciations, named Möhlin, Habsburg, Hagenholz (uncertain, inadequately documented), Beringen, and Birrfeld after their key regions. The Möhlin Glaciation represents the most extensive glaciation of the Swiss alpine foreland while the Beringen Glaciation had a slightly lesser extent. The last glacial cycle (Birrfeld Glaciation) probably comprises three independent glacial advances dated to ca. -

Behavior of Organic Compounds During Infiltration of River Water to Groundwater
Environ. Sci. Technol. 1983, 17, 472-479 Behavior of Organic Compounds during Infiltration of River Water to Groundwater. Field Studies Red P. Schwarzenbach,” Walter Giger, Eduard Hoehn,+ and Jurg K. Schnelder Swiss Federal Institute for Water Resources and Water Pollution Control (EAWAG), CH-8600 Dubendorf, Switzerland fields of two rivers, a network of observation wells was The behavior of organic micropollutants during infil- installed that allowed the contaminants in the infiltrating tration of river water to groundwater has been studied at two field sites in Switzerland. In agreement with predic- water to be traced from the river to the groundwater. The tions from model calculations, persistent organic chemicals results of this 2-year field study contribute significantly exhibiting octanol/water partition coefficients smaller than to the limited field data on the behavior of trace organics about 5000 moved rapidly with the infiltrating river water in the groundwater environment (9-11). to the groundwater. The biological processes responsible for the “elimination” of various micropollutants (e.g., al- Theoretical Section kylated and chlorinated benzenes) occurred predominantly Prediction of Retardation Factors for Hydrophobic within the first few meters of infiltration. Alkylated Organic Compounds in the Ground. A rough estimate benzenes were “eliminated” at faster rates than 1,4-di- of the retention behavior of a given hydrophobic organic chlorobenzene. Anaerobic conditions in the aquifer near compound during infiltration may be obtained by treating the river hindered the biological transformation of 1,4- transport through the river bed and in the aquifer in a first dichlorobenzene. Among the compounds that were found approximation as a one-dimensional process with constant to be persistent under any conditions were chloroform, flow in a homogeneous porous medium. -

Dem Fischrückgang Auf Der Spur
SCHLUSSBERICHT DES PROJEKTS NETZWERK FISCHRÜCKGANG SCHWEIZ – «FISCHNETZ» DEM FISCHRÜCKGANG AUF DER SPUR Januar 2004 Impressum Fischnetz Schlussbericht Herausgeberin Trägerschaft des Projekts «Fischnetz»: Eidgenössische Anstalt für Wasserversorgung, Abwasserreinigung und Gewässerschutz (EAWAG) Bundesamt für Umwelt, Wald und Landschaft (BUWAL) Fürstentum Liechtenstein (FL) und alle Kantone: Aargau (AG), Appenzell Innerrhoden (AI), Appenzell Ausserrhoden (AR), Bern (BE), Basel-Landschaft (BL), Basel-Stadt (BS), Freiburg (FR), Genf (GE), Glarus (GL), Graubünden (GR), Jura (JU), Luzern (LU), Neuenburg (NE), Nidwalden (NW), Obwalden (OW), St. Gallen (SG), Schaffhausen (SH), Solothurn (SO), Schwyz (SZ), Thurgau (TG), Tessin (TI), Uri (UR), Waadt (VD), Wallis (VS), Zug (ZG), Zürich (ZH) Schweizerische Gesellschaft für Chemische Industrie (SGCI) Schweizerischer Fischerei-Verband (SFV) Zentrum für Fisch- und Wildtiermedizin (FIWI), Universität Bern Universität Basel © Projekt Fischnetz, 2004 Text Projektleitung Fischnetz Journalistische Bearbeitung Claudia von See, Mannheim Redaktion Monika Meili, EAWAG Karin Scheurer, EAWAG Ori Schipper, EAWAG Patricia Holm, EAWAG und Universität Basel Grafiken Yvonne Lehnhard, EAWAG Illustrationen Karin Seiler, Zürich unter Verwendung von Fotos von Patricia Holm, Patrick Faller, Thomas Wahli, Matthias Escher, Eva Schager, ARA Surental Satz und Layout Peter Nadler, Küsnacht Druck Mattenbach AG, Winterthur Auflage 1000 Exemplare Hinweis Dieser Bericht wird auch in französischer Sprache erscheinen. Bezug EAWAG, Postfach 611, CH-8600 Dübendorf, Tel. +41 1 823 50 32, www.fischnetz.ch BUWAL, Dokumentation, CH-3003 Bern, Fax +41 (0)31 324 02 16, [email protected], www.buwalshop.ch Fischnetz-Schlussbericht Vorwort Vorwort Im Januar 1998 trafen sich die Vertreter von kantonalen fischereibiologischen und ökologischen Fragen Auskunft ge- Fischereibehörden, um die Entwicklung bei den Fischfängen geben. Wichtig sind ebenso der fortzusetzende Dialog mit zu besprechen. -
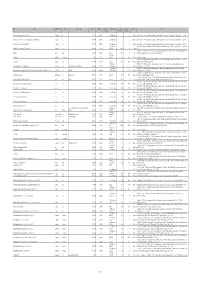
Available As PDF
ID Locality Administrative unit Country Alternate spelling Latitude Longitude Geographic Stratigraphic age Age, lower Age, upper Epoch Reference precision boundary boundary 3247 Acquasparta (along road to Massa Martana) Acquasparta Italy 42.707778 12.552333 1 late Villafranchian 2 1.1 Pleistocene Esu, D., Girotti, O. 1975. La malacofauna continentale del Plio-Pleistocene dell’Italia centrale. I. Paleontologia. Geologica Romana, 13, 203-294. 3246 Acquasparta (NE of 'km 32', below Chiesa di Santa Lucia di Burchiano) Acquasparta Italy 42.707778 12.552333 1 late Villafranchian 2 1.1 Pleistocene Esu, D., Girotti, O. 1975. La malacofauna continentale del Plio-Pleistocene dell’Italia centrale. I. Paleontologia. Geologica Romana, 13, 203-294. 3234 Acquasparta (Via Tiberina, 'km 32,700') Acquasparta Italy 42.710556 12.549556 1 late Villafranchian 2 1.1 Pleistocene Esu, D., Girotti, O. 1975. La malacofauna continentale del Plio-Pleistocene dell’Italia centrale. I. Paleontologia. Geologica Romana, 13, 203-294. upper Alluvial Ewald, R. 1920. Die fauna des kalksinters von Adelsheim. Jahresberichte und Mitteilungen des Oberrheinischen geologischen Vereines, Neue Folge, 3330 Adelsheim (Adelsheim, eastern part of the city) Adelsheim Germany 49.402305 9.401456 2 (Holocene) 0.00585 0 Holocene 9, 15-17. Sanko, A.F. 2007. Quaternary freshwater mollusks Belarus and neighboring regions of Russia, Lithuania, Poland (field guide). [in Russian]. Institute 5200 Adrov Adrov Belarus 54.465816 30.389993 2 Holocene 0.0117 0 Holocene of Geochemistry and Geophysics, National Academy of Sciences, Belarus. middle-late 4441 Adzhikui Adzhikui Turkmenistan 39.76667 54.98333 2 Pleistocene 0.781 0.0117 Pleistocene FreshGEN team decision Hagemann, J. 1976. Stratigraphy and sedimentary history of the Upper Cenozoic of the Pyrgos area (Western Peloponnesus), Greece. -

Distribution, Geometry, Age and Origin of Overdeepened Valleys and Basins in the Alps and Their Foreland
Swiss J Geosci (2010) 103:407–426 DOI 10.1007/s00015-010-0044-y Distribution, geometry, age and origin of overdeepened valleys and basins in the Alps and their foreland Frank Preusser • Ju¨rgen M. Reitner • Christian Schlu¨chter Received: 22 February 2010 / Accepted: 14 June 2010 / Published online: 14 December 2010 Ó Swiss Geological Society 2010 Abstract Overdeepened valleys and basins are commonly and excavated by glaciers during past glaciations. However, found below the present landscape surface in areas that were the age of the original formation of (non-Messinian) over- affected by Quaternary glaciations. Overdeepened troughs deepened structures remains unknown. The mechanisms and their sedimentary fillings are important in applied causing overdeepening also remain unidentified and it can geology, for example, for geotechnics of deep foundations only be speculated that pressurised meltwater played an and tunnelling, groundwater resource management, and important role in this context. radioactive waste disposal. This publication is an overview of the areal distribution and the geometry of overdeepened Keywords Glaciations Á Erosion Á Sediments Á troughs in the Alps and their foreland, and summarises the Overdeepening Á Alps Á Quaternary present knowledge of the age and potential processes that may have caused deep erosion. It is shown that overdee- pened features within the Alps concur mainly with tectonic Introduction structures and/or weak lithologies as well as with Pleisto- cene ice confluence and partly also diffluence situations. In ‘‘Mit Butter hobelt man nicht (Butter is not an abrasive the foreland, overdeepening is found as elongated buried agent)’’. This famous statement by Albert Heim (1919), valleys, mainly oriented in the direction of former ice flow, probably one of the most prominent alpine geologists of the and glacially scoured basins in the ablation area of glaciers.