Bacterial Communities in the Glenmore Reservoir with an Emphasis on the Cyanobacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rbcl Marker Based Approach for Molecular Identification of Arthrospira and Dunaliella Isolates from Non-Axenic Cultures
Journal of Genetics and Genetic Engineering Volume 2, Issue 2, 2018, PP 24-34 ISSN 2637-5370 Rbcl Marker Based Approach for Molecular Identification of Arthrospira and Dunaliella Isolates from Non-Axenic Cultures Akash Patel 1&2, Spandan Chaudhary3, Bakhtiyar Alam Syed2, Bharat Gami1, Pankaj Patel1, Beena Patel1* 1R & D Department, Abellon CleanEnergy Pvt. Ltd. Sydney House, Premchand Nagar Road, Ahmedabad-380054 INDIA 2Department of Biotechnology, Hemchandracharya North Gujarat University, Patan 384265, Gujarat, India. 3Xcelris Labs Limited 2nd Floor, Heritage Profile, 22-23, Shrimali Society, Opp. Navrangpura Police Station, Navrangpura, Ahmedabad - 380009, Gujarat, INDIA *Corresponding Author: Beena Patel,R & D Department, Abellon Clean Energy Pvt. Ltd. Sydney House, Premchand Nagar Road, Ahmedabad-380054 INDIA. [email protected] ABSTRACT Population escalation and energy crises have increased the urge to find renewable energy sources with higher sustainability index. Microalgae are potential candidates for biofuel feedstock production without utilizing fertile land and drinking water owing to their efficient oil production pathways and high value bio molecules. Morphological identification of potential microalgae species needs accurate molecular validation due to the versatility in nature, however PCR based molecular identification requires pure and axenic culture of isolates in universal primers based gene targets. In present study, monospecies algal culture were directly used to extract DNA followed by PCR amplification of 18S rDNA gene target using universal primers ITS1F-4R and rbcL gene target using primers designed for species specific gene. rbcL primers successfully amplified specific microalgae gene. Resulting sequences were annotated using multiple sequence alignment with Genebank databse and phylogenetic relationship study. These rbcL gene primers and validated PCR conditions can be used for non-axenic monospecies green microalgae isolates for easy and efficient molecular identification. -

Oscillatoriales, Microcoleaceae), Nuevo Reporte Para El Perú
Montoya et al.: Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Arnaldoa 24 (1): 369 - 382, 2017 ISSN: 1815-8242 (edición impresa) http://doi.org/10.22497/arnaldoa.241.24119 ISSN: 2413-3299 (edición online) Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Phenotypic diversity of the cyanobacterium Pseudo- phormidium tenue (Oscillatoriales, Microcoleaceae), new record for Peru Haydee Montoya T., José Gómez C., Mauro Mariano A., Enoc Jara P., Egma Mayta H., Mario Benavente P. Museo de Historia Natural, Departamento de Simbiosis Vegetal, UNMSM. Av. Arenales 1256. Apartado 14-0434. Lima 14, PERÚ. Instituto de Investigación de Ciencias Biológicas, Facultad de CC. Biológicas, UNMSM [email protected], [email protected], [email protected] [email protected] 24 (1): Enero - Junio, 2017 369 Este es un artículo de acceso abierto bajo la licencia CC BY-NC 4.0: https://creativecommons.org/licenses/by-nc/4.0/ Montoya et al.: Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Recibido: 20-I-2017; Aceptado: 15-III-2017; Publicado: VI-2017; Edición online: 05-VI-2017 Resumen Los ecosistemas desérticos costeros tropicales están distribuidos ampliamente en el oeste de Sudamérica. No obstante las tierras áridas de esta región, la disponibilidad hídrica de la humedad proveniente de las neblinas a nivel del océano Pacífico acarreadas hacia las colinas (lomas) y las garúas anuales invernales fluctuantes favorecen el desarrollo de comunidades cianobacteriales extremas. El área de evaluación fue las Lomas de Pachacámac, al sur de Lima, y las colecciones cianobacteriales estándar (costras, biofilms o matas terrestres) fueron realizadas irregularmente en 1995 y 2012. -

Akashiwo Sanguinea
Ocean ORIGINAL ARTICLE and Coastal http://doi.org/10.1590/2675-2824069.20-004hmdja Research ISSN 2675-2824 Phytoplankton community in a tropical estuarine gradient after an exceptional harmful bloom of Akashiwo sanguinea (Dinophyceae) in the Todos os Santos Bay Helen Michelle de Jesus Affe1,2,* , Lorena Pedreira Conceição3,4 , Diogo Souza Bezerra Rocha5 , Luis Antônio de Oliveira Proença6 , José Marcos de Castro Nunes3,4 1 Universidade do Estado do Rio de Janeiro - Faculdade de Oceanografia (Bloco E - 900, Pavilhão João Lyra Filho, 4º andar, sala 4018, R. São Francisco Xavier, 524 - Maracanã - 20550-000 - Rio de Janeiro - RJ - Brazil) 2 Instituto Nacional de Pesquisas Espaciais/INPE - Rede Clima - Sub-rede Oceanos (Av. dos Astronautas, 1758. Jd. da Granja -12227-010 - São José dos Campos - SP - Brazil) 3 Universidade Estadual de Feira de Santana - Departamento de Ciências Biológicas - Programa de Pós-graduação em Botânica (Av. Transnordestina s/n - Novo Horizonte - 44036-900 - Feira de Santana - BA - Brazil) 4 Universidade Federal da Bahia - Instituto de Biologia - Laboratório de Algas Marinhas (Rua Barão de Jeremoabo, 668 - Campus de Ondina 40170-115 - Salvador - BA - Brazil) 5 Instituto Internacional para Sustentabilidade - (Estr. Dona Castorina, 124 - Jardim Botânico - 22460-320 - Rio de Janeiro - RJ - Brazil) 6 Instituto Federal de Santa Catarina (Av. Ver. Abrahão João Francisco, 3899 - Ressacada, Itajaí - 88307-303 - SC - Brazil) * Corresponding author: [email protected] ABSTRAct The objective of this study was to evaluate variations in the composition and abundance of the phytoplankton community after an exceptional harmful bloom of Akashiwo sanguinea that occurred in Todos os Santos Bay (BTS) in early March, 2007. -
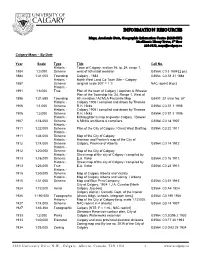
Information Resources
INFORMATION RESOURCES Maps, Academic Data, Geographic Information Centre (MADGIC) MacKimmie Library Tower, 2nd floor 220-8132, [email protected] Calgary Maps – By Date Year Scale Type Title Call No. Historic - Town of Calgary, section 16, tp. 24, range 1, 1884 1:3,000 Scheme west of 5th initial meridian G3564 .C3 3 1884 [2 pc] 1884 1:31,000 Township Calgary - 1883 G3564 .C3 S1 31 1884 Historic - North West Land Co Town Site – Calgary 1887 Scheme [original scale 300’ = 1 “] NAC reprint [4 pc] Historic - 1891 1:6,000 True Plan of the town of Calgary / Jepshon & Wheeler Plan of the Township No. 24, Range 1, West of 1895 1:31,680 Township 5th meridian / ACMLA Facsimile Map G3401 .S1 sVar No. 57 Historic - Calgary 1906 / compiled and drawn by Thomas 1906 1:1,000 Scheme R.H. Hicks G3564 .C3 S1 1 1906 Historic - Calgary 1906 / compiled and drawn by Thomas 1906 1:3,000 Scheme R.H. Hicks G3564 .C3 S1 3 1906 Historic - McNaughton's map of greater Calgary / Dowler 1907 1:14,000 Scheme & Michie architects & compilers. G3564 .C3 14 1907 Historic - 1911 1:22,000 Scheme Plan of the City of Calgary / Great West Drafting G3564 .C3 22 1911 Historic - 1911 1:34,000 Scheme Map of the City of Calgary Historic - Harrison and Ponton's map of the City of 1912 1:14,000 Scheme Calgary, Province of Alberta G3564 .C3 14 1912 Historic - 1912 1:20,000 Scheme Map of the City of Calgary Historic - Street map of the city of Calgary / compiled by 1913 1:16,000 Scheme E.A. -

Protocols for Monitoring Harmful Algal Blooms for Sustainable Aquaculture and Coastal Fisheries in Chile (Supplement Data)
Protocols for monitoring Harmful Algal Blooms for sustainable aquaculture and coastal fisheries in Chile (Supplement data) Provided by Kyoko Yarimizu, et al. Table S1. Phytoplankton Naming Dictionary: This dictionary was constructed from the species observed in Chilean coast water in the past combined with the IOC list. Each name was verified with the list provided by IFOP and online dictionaries, AlgaeBase (https://www.algaebase.org/) and WoRMS (http://www.marinespecies.org/). The list is subjected to be updated. Phylum Class Order Family Genus Species Ochrophyta Bacillariophyceae Achnanthales Achnanthaceae Achnanthes Achnanthes longipes Bacillariophyta Coscinodiscophyceae Coscinodiscales Heliopeltaceae Actinoptychus Actinoptychus spp. Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Akashiwo Akashiwo sanguinea Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Amphidinium Amphidinium spp. Ochrophyta Bacillariophyceae Naviculales Amphipleuraceae Amphiprora Amphiprora spp. Bacillariophyta Bacillariophyceae Thalassiophysales Catenulaceae Amphora Amphora spp. Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Anabaenopsis Anabaenopsis milleri Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema Anagnostidinema amphibium Anagnostidinema Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema lemmermannii Cyanobacteria Cyanophyceae Oscillatoriales Microcoleaceae Annamia Annamia toxica Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Aphanizomenon Aphanizomenon flos-aquae -

Planktothrix Agardhii É a Mais Comum
Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters Catarina Isabel Prata Pereira Leitão Churro Doutoramento em Biologia Departamento Biologia 2015 Orientador Vitor Manuel de Oliveira e Vasconcelos, Professor Catedrático Faculdade de Ciências iv FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters The research presented in this thesis was supported by the Portuguese Foundation for Science and Technology (FCT, I.P.) national funds through the project PPCDT/AMB/67075/2006 and through the individual Ph.D. research grant SFRH/BD65706/2009 to Catarina Churro co-funded by the European Social Fund (Fundo Social Europeu, FSE), through Programa Operacional Potencial Humano – Quadro de Referência Estratégico Nacional (POPH – QREN) and Foundation for Science and Technology (FCT). The research was performed in the host institutions: National Institute of Health Dr. Ricardo Jorge (INSA, I.P.), Lisboa; Interdisciplinary Centre of Marine and Environmental Research (CIIMAR), Porto and Centre for Microbial Resources (CREM - FCT/UNL), Caparica that provided the laboratories, materials, regents, equipment’s and logistics to perform the experiments. v FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters vi FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters ACKNOWLEDGMENTS I would like to express my gratitude to my supervisor Professor Vitor Vasconcelos for accepting to embark in this research and supervising this project and without whom this work would not be possible. I am also greatly thankful to my co-supervisor Elisabete Valério for the encouragement in pursuing a graduate program and for accompanying me all the way through it. -

RURAL ECONOMY Ciecnmiiuationofsiishiaig Activity Uthern All
RURAL ECONOMY ciEcnmiIuationofsIishiaig Activity uthern All W Adamowicz, P. BoxaIl, D. Watson and T PLtcrs I I Project Report 92-01 PROJECT REPORT Departmnt of Rural [conom F It R \ ,r u1tur o A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta W. Adamowicz, P. Boxall, D. Watson and T. Peters Project Report 92-01 The authors are Associate Professor, Department of Rural Economy, University of Alberta, Edmonton; Forest Economist, Forestry Canada, Edmonton; Research Associate, Department of Rural Economy, University of Alberta, Edmonton and Research Associate, Department of Rural Economy, University of Alberta, Edmonton. A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta Interim Project Report INTROI)UCTION Recreational fishing is one of the most important recreational activities in Alberta. The report on Sports Fishing in Alberta, 1985, states that over 340,000 angling licences were purchased in the province and the total population of anglers exceeded 430,000. Approximately 5.4 million angler days were spent in Alberta and over $130 million was spent on fishing related activities. Clearly, sportsfishing is an important recreational activity and the fishery resource is the source of significant social benefits. A National Angler Survey is conducted every five years. However, the results of this survey are broad and aggregate in nature insofar that they do not address issues about specific sites. It is the purpose of this study to examine in detail the characteristics of anglers, and angling site choices, in the Southern region of Alberta. Fish and Wildlife agencies have collected considerable amounts of bio-physical information on fish habitat, water quality, biology and ecology. -

South Saskatchewan River Basin Adaptation to Climate Variability Project
South Saskatchewan River Basin Adaptation to Climate Variability Project Climate Variability and Change in the Bow River Basin Final Report June 2013 This study was commissioned for discussion purposes only and does not necessarily reflect the official position of the Climate Change Emissions Management Corporation, which is funding the South Saskatchewan River Basin Adaptation to Climate Variability Project. The report is published jointly by Alberta Innovates – Energy and Environment Solutions and WaterSMART Solutions Ltd. This report is available and may be freely downloaded from the Alberta WaterPortal website at www.albertawater.com. Disclaimer Information in this report is provided solely for the user’s information and, while thought to be accurate, is provided strictly “as is” and without warranty of any kind. The Crown, its agents, employees or contractors will not be liable to you for any damages, direct or indirect, or lost profits arising out of your use of information provided in this report. Alberta Innovates – Energy and Environment Solutions (AI-EES) and Her Majesty the Queen in right of Alberta make no warranty, express or implied, nor assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information contained in this publication, nor that use thereof infringe on privately owned rights. The views and opinions of the author expressed herein do not necessarily reflect those of AI-EES or Her Majesty the Queen in right of Alberta. The directors, officers, employees, agents and consultants of AI-EES and the Government of Alberta are exempted, excluded and absolved from all liability for damage or injury, howsoever caused, to any person in connection with or arising out of the use by that person for any purpose of this publication or its contents. -

This Collaborative Workshop by Cows & Fish and the Alberta Low Impact
From Street to Stream - this collaborative workshop by Cows & Fish and the Alberta Low Impact Development Partnership (ALIDP) explores how water quality, stormwater runoff, natural water storage and resiliency can be improved in developed areas. In this workshop series we look at low impact development, sound riparian restoration and management, and the importance of considering both upland and riparian areas as part of a whole system management approach. This workshop series is geared for the public, realtors, decision makers (planners, municipalities), green builders, and others interested in sustainability related to stormwater, greening, housing, and landscapes. 1 This workshop series builds on a Natural Capital workshop offered in 2010 by Cows and Fish for the Alberta Real Estate Foundation where we explored the Environmental Impacts on Real Estate Values & Marketing. 2 The primary sponsors for our current Street to Stream workshop include the Alberta Real Estate Foundation, The Calgary Foundation and The RBC Blue Water Project. Thank you also to our local sponsors and co-hosts for our six Street to Stream workshops conducted in Calgary, Red Deer, Edmonton, Wetaskiwin and Lethbridge from February to April, 2015. 3 I am here today representing the Alberta Riparian Habitat Management Society or more commonly known as Cows and Fish. Cows and Fish is a charitable, non-profit society based in Alberta. For over 20 years, with the help of our members and supporters listed on this slide, we have been working with landowners and community groups across the province to promote and foster riparian stewardship. As our name suggests, we have our roots in tackling agricultural issues, but more and more we are also working with urban and lakeshore riparian stewardship groups. -

Hydrology Study, Bow and Elbow River Updated Hydraulic Model Project, Rev
March 2010 HYDROLOGY STUDY, BOW AND ELBOW RIVER UPDATED HYDRAULIC MODEL PROJECT, REV. A Submitted to: Alberta Environment REPORT Report Number: 09-1326-1040 HYDROLOGY STUDY, BOW AND ELBOW RIVER UPDATED HYDRAULIC MODEL PROJECT Executive Summary Golder Associates Ltd. (Golder) was commissioned by Alberta Environment (AENV) to conduct a hydrology study for the “Bow and Elbow River Updated Hydraulic Model” project. The City of Calgary (the City), in partnership with Alberta Environment (AENV), plans to create a HEC-RAS (Hydraulic Engineering Center River Analysis System) hydraulic model of the Bow and Elbow Rivers through the City. The model implementation is primarily for supporting emergency response planning and operations through flood inundation mapping. It will provide additional perspective on current flood hazard area management and will provide a basis for increased understanding of fish habitat, river morphology and erosion, water quality and storm water runoff impacts. The scope of work for the hydrology study included: 1) Generation of naturalized daily flow series at the major storage facilities on the Bow River above Bearspaw Dam and on the Elbow River at Glenmore Reservoir; 2) Estimation of naturalized and regulated 1:2 to 1:1,000 year flood flows based on flood frequency analysis of naturalized and/or recorded peak flow series at relevant locations along the Bow River and its tributaries (including the Elbow River); 3) Development of synthetic inflow flood hydrographs for tributaries to the Bow River with hydropower developments and at the Water Survey of Canada (WSC) Bow River at Banff station; 4) Routing of the synthetic flood hydrographs through all major storage reservoirs along the Bow River above Bearspaw Dam and through the Glenmore Reservoir; 5) Commentary on the effects of climate change and the impact of stormwater runoff on flood estimates as well as on the seasonality of flood peaks; and 6) Comparison of the new flood flow estimates with those of AENV’s 1983 Calgary Floodplain Study (the 1983 study). -

First Insights Into the Impacts of Benthic Cyanobacterial Mats on Fish
www.nature.com/scientificreports OPEN First insights into the impacts of benthic cyanobacterial mats on fsh herbivory functions on a nearshore coral reef Amanda K. Ford 1,2*, Petra M. Visser 3, Maria J. van Herk3, Evelien Jongepier 4 & Victor Bonito5 Benthic cyanobacterial mats (BCMs) are becoming increasingly common on coral reefs. In Fiji, blooms generally occur in nearshore areas during warm months but some are starting to prevail through cold months. Many fundamental knowledge gaps about BCM proliferation remain, including their composition and how they infuence reef processes. This study examined a seasonal BCM bloom occurring in a 17-year-old no-take inshore reef area in Fiji. Surveys quantifed the coverage of various BCM-types and estimated the biomass of key herbivorous fsh functional groups. Using remote video observations, we compared fsh herbivory (bite rates) on substrate covered primarily by BCMs (> 50%) to substrate lacking BCMs (< 10%) and looked for indications of fsh (opportunistically) consuming BCMs. Samples of diferent BCM-types were analysed by microscopy and next-generation amplicon sequencing (16S rRNA). In total, BCMs covered 51 ± 4% (mean ± s.e.m) of the benthos. Herbivorous fsh biomass was relatively high (212 ± 36 kg/ha) with good representation across functional groups. Bite rates were signifcantly reduced on BCM-dominated substratum, and no fsh were unambiguously observed consuming BCMs. Seven diferent BCM-types were identifed, with most containing a complex consortium of cyanobacteria. These results provide insight into BCM composition and impacts on inshore Pacifc reefs. Tough scarcely mentioned in the literature a decade ago, benthic cyanobacterial mats (BCMs) are receiving increasing attention from researchers and managers as being a nuisance on tropical coral reefs worldwide1–4. -

Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection
toxins Review Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection Isidro José Tamele 1,2,3 and Vitor Vasconcelos 1,4,* 1 CIIMAR/CIMAR—Interdisciplinary Center of Marine and Environmental Research, University of Porto, Terminal de Cruzeiros do Porto, Avenida General Norton de Matos, 4450-238 Matosinhos, Portugal; [email protected] 2 Institute of Biomedical Science Abel Salazar, University of Porto, R. Jorge de Viterbo Ferreira 228, 4050-313 Porto, Portugal 3 Department of Chemistry, Faculty of Sciences, Eduardo Mondlane University, Av. Julius Nyerere, n 3453, Campus Principal, Maputo 257, Mozambique 4 Faculty of Science, University of Porto, Rua do Campo Alegre, 4069-007 Porto, Portugal * Correspondence: [email protected]; Tel.: +351-223-401-817; Fax: +351-223-390-608 Received: 6 May 2020; Accepted: 31 May 2020; Published: 2 June 2020 Abstract: Microcystins (MCs) are cyanotoxins produced mainly by freshwater cyanobacteria, which constitute a threat to public health due to their negative effects on humans, such as gastroenteritis and related diseases, including death. In Mozambique, where only 50% of the people have access to safe drinking water, this hepatotoxin is not monitored, and consequently, the population may be exposed to MCs. The few studies done in Maputo and Gaza provinces indicated the occurrence of MC-LR, -YR, and -RR at a concentration ranging from 6.83 to 7.78 µg L 1, which are very high, around 7 times · − above than the maximum limit (1 µg L 1) recommended by WHO. The potential MCs-producing in · − the studied sites are mainly Microcystis species.