The Impact of Health Reform on Health System Spending
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

China's Health System Reform and Global Health Strategy in The
Testimony China’s Health System Reform and Global Health Strategy in the Context of COVID-19 Jennifer Bouey CT-A321-1 Testimony presented before the U.S.-China Economic and Security Review Commission on May 7, 2020. C O R P O R A T I O N For more information on this publication, visit www.rand.org/pubs/testimonies/CTA321-1.html Testimonies RAND testimonies record testimony presented or submitted by RAND associates to federal, state, or local legislative committees; government-appointed commissions and panels; and private review and oversight bodies. ChapterTitle 1 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2020 RAND Corporation RA® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. www.rand.org China’s Health System Reform and Global Health Strategy in the Context of COVID-19 Testimony of Jennifer Bouey1 The RAND Corporation2 Before the U.S.-China Economic and Security Review Commission May 7, 2020 hairman Cleveland, Commissioner Lee, and members of the Commission, thank you for inviting me to assess China’s pandemic-related issues regarding its public health C system, health care system, and global health strategy in the context of COVID-19. -

The French Health Care System
The french health care system The french health care system VICTOR RODWIN PROFESSOR OF HEALTH POLICY AND MANAGEMENT WAGNER SCHOOL OF PUBLIC SERVICE, NEW YORK UNIVERSITY NEW YORK, USA ABSTRACT: The French health care system is a model of national health insurance (NHI) that provides health care coverage to all legal residents. It is an example of public social security and private health care financing, combined with a public-private mix in the provision of health care services. The French health care system reflects three underlying political values: liberalism, pluralism and solidarity. This article provides a brief overview of how French NHI evolved since World War II; its financing health care organization and coverage; and most importantly, its overall performance. ntroduction. Evolution, coverage, financing and organization The French health care system is a model of national health Evolution: French NHI evolved in stages and in response to Iinsurance (NHI) that provides health care coverage to all legal demands for extension of coverage. Following its original passage, residents. It is not an example of socialized medicine, e.g. Cuba. in 1928, the NHI program covered salaried workers in industry and It is not an example of a national health service, as in the United commerce whose wages were under a low ceiling (Galant, 1955). Kingdom, nor is it an instance of a government-run health care In 1945, NHI was extended to all industrial and commercial workers system like the United States Veterans Health Administration. and their families, irrespective of wage levels. The extension of French NHI, in contrast, is an example of public, social security and coverage took the rest of the century to complete. -

Expensive Patients, Reinsurance, and the Future of Health Care Reform
Emory Law Journal Volume 69 Issue 6 2020 Expensive Patients, Reinsurance, and the Future of Health Care Reform Govind Persad Follow this and additional works at: https://scholarlycommons.law.emory.edu/elj Recommended Citation Govind Persad, Expensive Patients, Reinsurance, and the Future of Health Care Reform, 69 Emory L. J. 1153 (2020). Available at: https://scholarlycommons.law.emory.edu/elj/vol69/iss6/1 This Article is brought to you for free and open access by the Journals at Emory Law Scholarly Commons. It has been accepted for inclusion in Emory Law Journal by an authorized editor of Emory Law Scholarly Commons. For more information, please contact [email protected]. PERSAD_8.21.20 8/24/2020 11:43 AM EXPENSIVE PATIENTS, REINSURANCE, AND THE FUTURE OF HEALTH CARE REFORM Govind Persad* ABSTRACT In 2017, Americans spent over $3.4 trillion—nearly 18% of gross domestic product—on health care. This spending is unevenly distributed: Almost a quarter is spent on the costliest 1% of patients, and almost half on the costliest 5%. Most of these patients soon return to a lower percentile, but many continue to incur health care costs in the top percentiles year after year. This Article focuses on the challenges that persistently expensive patients present for health law and policy, and how fairly dividing their medical costs among payers illuminates fundamental normative choices about the design and reform of health insurance. In doing so, this Article draws on bioethical and health policy analyses of the fair distribution of medical costs, and examines how legal doctrine shapes health systems’ options for responding to expensive patients. -

Connecticut Medicaid: a Primer July 2010
Connecticut Medicaid: A Primer July 2010 By Signe Peterson Flieger, M.S.W. Medicaid Overview Medicaid has provided a health care safety net to millions of Americans since its enactment under Title XIX of the Social Security Act in 1965. In 2007, Medicaid provided health care coverage for almost 60 million Americans.1 With the recent passage of the national health reform law, the Patient Protection and Affordable Care Act (PPACA), Medicaid will continue to play a significant role in the expansion of health insurance coverage to many Americans. In its current form, Medicaid provides health care coverage for many low-income children and families who do not have access to employer-sponsored insurance, individuals with disabilities who lack private coverage or for whom adequate coverage is not available, and low-income seniors dually eligible for both Medicare and Medicaid.1 In addition, Medicaid is the largest payer of long-term care services, and finances more than 40 percent of overall nursing home and long-term care spending, including both institutional care and home and community-based services.1 Medicaid is jointly financed by the state and federal government as an entitlement program. States receive federal matching dollars at a rate based on state per capita income, with poorer states receiving more federal money. In contrast, the Children’s Health Insurance Program (CHIP) is a block grant program, so coverage can be denied for eligible children when the funding runs out.1 States receive higher federal matching rates for CHIP than for Medicaid. Participation in Medicaid and CHIP is voluntary, but all states, the District of Columbia, and the territories participate. -

Addressing Health Care Market Consolidation and High Prices the Role of the States
HEALTH POLICY CENTER RESEARCH REPORT Addressing Health Care Market Consolidation and High Prices The Role of the States Robert A. Berenson Jaime S. King Katherine L. Gudiksen Roslyn Murray URBAN INSTITUTE UC HASTINGS LAW UC HASTINGS LAW GEORGETOWN UNIVERSITY Adele Shartzer URBAN INSTITUTE January 2020 ABOUT THE URBAN INSTITUTE The nonprofit Urban Institute is a leading research organization dedicated to developing evidence-based insights that improve people’s lives and strengthen communities. For 50 years, Urban has been the trusted source for rigorous analysis of complex social and economic issues; strategic advice to policymakers, philanthropists, and practitioners; and new, promising ideas that expand opportunities for all. Our work inspires effective decisions that advance fairness and enhance the well-being of people and places. Copyright © January 2020. Urban Institute. Permission is granted for reproduction of this file, with attribution to the Urban Institute. Cover image by Tim Meko. Contents Acknowledgments iv Executive Summary v 1. Introduction 1 2. Employee Retirement Income Security’s Act 8 3. State Efforts to Promote Transparency 11 4. State Efforts to Regulate Consolidation and Promote Competition 17 5. State Options for Overseeing and Regulating Prices 44 6. Conclusion 68 Notes 69 References 79 About the Authors 86 Statement of Independence 87 Acknowledgments This report was funded by the Commonwealth Fund. We are grateful to them and to all our funders, who make it possible for Urban to advance its mission. The views expressed are those of the authors and should not be attributed to the Urban Institute or UC Hastings Law, its trustees, or its funders. -

Hand Hygiene: Clean Hands for Healthcare Personnel
Core Concepts for Hand Hygiene: Clean Hands for Healthcare Personnel 1 Presenter Russ Olmsted, MPH, CIC Director, Infection Prevention & Control Trinity Health, Livonia, MI Contributions by Heather M. Gilmartin, NP, PhD, CIC Denver VA Medical Center University of Colorado Laraine Washer, MD University of Michigan Health System 2 Learning Objectives • Outline the importance of effective hand hygiene for protection of healthcare personnel and patients • Describe proper hand hygiene techniques, including when various techniques should be used 3 Why is Hand Hygiene Important? • The microbes that cause healthcare-associated infections (HAIs) can be transmitted on the hands of healthcare personnel • Hand hygiene is one of the MOST important ways to prevent the spread of infection 1 out of every 25 patients has • Too often healthcare personnel do a healthcare-associated not clean their hands infection – In fact, missed opportunities for hand hygiene can be as high as 50% (Chassin MR, Jt Comm J Qual Patient Saf, 2015; Yanke E, Am J Infect Control, 2015; Magill SS, N Engl J Med, 2014) 4 Environmental Surfaces Can Look Clean but… • Bacteria can survive for days on patient care equipment and other surfaces like bed rails, IV pumps, etc. • It is important to use hand hygiene after touching these surfaces and at exit, even if you only touched environmental surfaces Boyce JM, Am J Infect Control, 2002; WHO Guidelines on Hand Hygiene in Health Care, WHO, 2009 5 Hands Make Multidrug-Resistant Organisms (MDROs) and Other Microbes Mobile (Image from CDC, Vital Signs: MMWR, 2016) 6 When Should You Clean Your Hands? 1. Before touching a patient 2. -
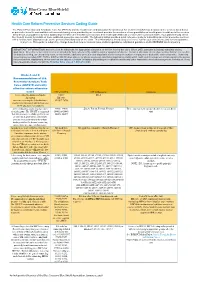
Health Care Reform Preventive Services Coding Guide
Health Care Reform Preventive Services Coding Guide The Patient Protection and Affordable Care Act (PPACA) and the Health Care and Education Reconciliation Act of 2010 (HCERA) has designated the services listed below as preventive benefits and available with no cost-sharing when provided by an in-network provider for members of non-grandfathered health plans. In addition to the services listed below, your patient may have additional preventive care benefits covered under their health plan that may or may not be covered at 100%. Your patient should check their benefit booklet for details on these additional preventive care benefits. The following tables provide a quick reference guide for submitting claims for preventive services with a “well-person” diagnosis code as the primary (first) diagnosis on the claim. This information is intended as a reference tool for your convenience and is not a guarantee of payment. This guide is subject to change based on new or revised laws and/or regulations, additional guidance and/or BCBSNC medical policy. IMPORTANT INFORMATION: Services must be billed with the appropriate diagnosis, at the line level of the claim (Block 24E), pursuant to industry standard coding guidelines. Preventive or screening services are intended for those who currently exhibit no signs or symptoms of disease. Services otherwise deemed preventive that are received in an inpatient setting, an emergency room, or that include additional procedures or diagnostic services may be subject to copayment, deductible and coinsurance. Submitting screening service codes (CPT, HCPCs, ICD-9 or ICD-10) when signs or symptoms are present constitutes inappropriate coding which could result in recoupment of monies paid to the provider for those services. -

Chapter 9: Monitoring, Surveillance, and Investigation of Health Threats
Chapter 9: Monitoring, surveillance, and investigation of health threats SUMMARY POINTS · Monitoring, surveillance and investigation of health threats are vital capabilities for an effective health system. The International Health Regulations (2005) require countries to maintain an integrated, national system for public health surveillance and response, and set out the core capabilities that countries are required to achieve. · Systematic monitoring of serious infectious diseases and other conditions is typically achieved through notifiable diseases legislation based on clinical observation and laboratory confirmation. Clinical and laboratory-based surveillance also provides the basis for systematic collection of vital statistics (births, deaths, causes of death), and may extend to the reporting and analysis of risk factors for noncommunicable diseases and injuries. Systematic collection of these data informs the allocation of resources and facilitates evaluation of community-based and population-level prevention strategies. · Clinical and laboratory surveillance are passive systems that may be enhanced by sentinel surveillance and/or community-based surveillance strategies that rely on a wider range of people, including non-medical personnel. Suspected cases identified in this way must be treated with respect, protected from discrimination, with diagnosis confirmed by qualified health workers at the earliest opportunity. · A significant degree of stigma may attach to some diseases, such as HIV, sexually transmitted infections and diabetes. Notifiable diseases legislation should require the protection of personal information, and clearly define any exceptions. Concerns about discrimination and breach of privacy may be addressed by requiring certain diseases to be reported on an anonymous or de- identified basis. · In some countries, legislation or regulations may be used to establish or enhance a comprehensive public health surveillance system. -

Big Bang Health Care Reform — Does It Work?: the Case of Britain’S 1991 National Health Service Reforms
Big Bang Health Care Reform — Does It Work?: The Case of Britain’s 1991 National Health Service Reforms RUDOLF KLEIN University o f Bath, England ealth care reform has b e e n o n e of the worldwide epidemics of the 1990s. But among the many coun tries that have either attempted or contemplated the reform H of their health care systems (Hurst 1992; Organization for Economic Co-operation and Development 1994) Britain stands out from the rest. The reforms of the National Health Service (NHS) introduced in 1991 by Mrs. Thatcher’s Conservative government were driven by the much the same set of concerns and ideas that shaped the international vocabu lary of debate. In particular, they reflected the widely held belief that the best way of improving efficiency was to change the incentives to pro viders and that some form of marketlike competition was the best tool for achieving this aim (Saltman and van Otter 1992). In all these re spects, there was nothing all that special about Britain. What makes the British case special, and worthy of further study, however, is the ambi tious scope of the reforms and the relentless determination with which Mrs. Thatcher’s government implemented them. In the United States, the Clinton reforms plan foundered on the rocks of congressional oppo sition; in Sweden, a succession of local experiments and committees of inquiry failed to create a national consensus about the direction of health care policy; in the Netherlands, an ambitious plan of reform was The Milbank Quarterly, Vol. 73, No. 3, 1995 © 1995 Milbank Memorial Fund. -

Nutrition Informatics
Practice Paper of the Academy of Nutrition and Dietetics: Nutrition Informatics ABSTRACT As valued members and decision makers of the health for informatics practice, use of computers greatly facilitates care team, registered dietitians and dietetic technicians, management of large amounts of information. Health registered practice in a wide variety of settings from corporate informatics focuses on the application of information science wellness to the intensive care unit. Each of these work within the health care arena. The field of health informatics settings has unique information needs, but all require that includes medical, nursing, pharmacy, dental, public health, dietetics practitioners have immediate access to accurate and now nutrition informatics. Registered dietitians and information. Successful dietetics practice in today’s rapidly dietetic technicians, registered are now creating nutrition changing environment requires skills in finding, evaluating, informatics as a new area of dietetics practice. Current use of and sharing accurate food and nutrition information. The informatics in health care includes electronic health records, term informatics is used to describe the science of managing, outcomes research, and knowledge acquisition. storing, and communicating information. While not required There is no doubt that food and nutrition practitioners related problem solving and decision-making. Informatics manage large amounts of information on a daily basis. is supported by the use of information standards, processes, The term informatics is used to describe how humans and technology. Figure 2 illustrates some of the myriad ways find, store, analyze, and manage information. Health care that dietetics practitioners in all areas of practice utilize informatics was developed as an area of specialization within informatics tools. -

What Is Sustinet? of Interest
August 5, 2009 The Government Affairs Group at Murtha Cullina LLP is pleased to pro- vide information about current topics WHAT IS SUSTINET? of interest. David J. McQuade* 860.240.6141 By overriding Governor M. Jodi Rell’s veto of the SustiNet act, the Connecticut General As- [email protected] sembly took the first step in moving the state to a “system of universal healthcare” for all its Janemarie W. Murphy* citizens. As approved, Public Act 09-148, “An Act Concerning the Establishment of the Sus- 860.240.6143 [email protected] tiNet Plan,” calls for the creation of a new self-insured health plan to provide health services Michael J. Martone* to a gradually expanding pool of individuals in this state. Starting with the “voluntary” enroll- 860.240.6109 [email protected] ment of non-state public employees, nonprofit groups, and small businesses, the SustiNet Kylene A. Fredrick* Plan requires the state to include all clients of state supported health plans, including HUSKY 860.240.6040 A and B, Medicaid and the state-administered general assistance program; eventually ex- [email protected] panding services to state employees and retirees, the uninsured and larger employers. The Jason K. Crisco* 860.240.6185 act establishes July 1, 2012 as the target date for initial enrollments. [email protected] Establishment of the Plan * Not an Attorney Following a series of guidelines provided in the act, the SustiNet Plan will be developed by a nine-member board of directors. They are as follows: • The State Comptroller (serving -

Good Pharmacovigilance Practices for the Americas ISBN 978-92-75-13160-2 PANDRH Technical Document Nº 5 Pan American Network on Drug Regulatory Harmonization
PANDRH Technical Document Nº 5 Pan American Network on Drug Regulatory Harmonization PANDRH Technical Document Nº 5 Technical PANDRH Working Group on Pharmacovigilance Good Pharmacovigilance Good Pharmacovigilance Practices for the Americas Good Pharmacovigilance Practices for the Practices for the Americas ISBN 978-92-75-13160-2 PANDRH Technical Document Nº 5 Pan American Network on Drug Regulatory Harmonization Worki ng Group on Pharmacov iigil ilance Good Pharmacovigilance Practices for the Americas Washington, DC June 2011 PAHO Headquarters Library – Catalogued at the source Pan American Health Organization. “Good Pharmacovigilance Practices for the Americas”. Washington, D.C.: PAHO, © 2011. (PANDRH Technical Document Nº 5). 73 p. ISBN 978-92-75-13160-2 Published in Spanish with the title: “Buenas Prácticas de Farmacovigilancia para las Américas” Published in Portuguese with the title: “Boas práticas de farmacovigilância para as Américas” I Title 1. PHARMACOEPIDEMIOLOGY – legislation and jurisprudence 2. NATIONAL HEALTH SURVEILLANCE SYSTEM 3. PHARMACEUTICAL LEGISLATION – standards 4. DRUG LEGISLATION – economics 5. DRUGS AND NARCOTICS CONTROL 6. HEALTH POLICIES 7. AMERICAS NLM QV 733.DA1 Approved by the V PANDRH Conference in 2008. Validated in 2010. © Pan American Health Organization, 2011 All rights reserved. Requests for this publication should be directed to the Area of Health Systems based on Primary Health Care, Project on Essential Medicines and Technologies, Pan American Health Organization / World Health Organization, 525 23rd St., NW, Washington, D.C., USA [phone: +(202) 974-3483; e-mail: cas- [email protected]]. Requests for authorization to reproduce or translate PAHO publications—whether for sale or noncommercial distribution—should be directed to the Area of Knowledge Management and Communications (KMC) at the above address [fax: +(202) 974-3652; e-mail: [email protected]].