China's Health System Reform and Global Health Strategy in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Public Intellectuals Program
PUBLIC INTELLECTUALS PROGRAM The National Committee on United States-China Relations’ Public Intellectuals Program is designed to nurture a new generation of China specialists who have the interest and potential to play significant roles as public intellectuals. The goal of the program is to upgrade the quality of the American public’s understanding of China by strengthening links among U.S. academics, policymakers, and opinion leaders. The first three rounds of the program (2005-13) were funded with grants from The Henry Luce Foundation and The Starr Foundation. The fourth and fifth rounds of the program (2014-18) are funded by Carnegie Corporation of New York. Through a varied set of activities, the program helps twenty young American China scholars and other specialists deepen and broaden their knowledge about China’s politics, economics, and society, and encourages them to use this knowledge to inform policy and public opinion. The multi-year enrichment opportunity is intended to complement the fellows’ primary academic or professional positions. It includes two meetings in Washington focusing on the D.C.-based China policy community; a meeting in San Francisco; trips to China as a cohort; participation in National Committee programs as scholar-escorts; access to a media coach who can assist fellows in writing and placing op-eds; and a requirement that the fellows organize local public education programs. For more on the program, visit http://www.ncuscr.org/pip. PIP V FELLOWS: 2016-2018 Dr. Ang Yuen Yuen Assistant Professor, Political Science, University of Michigan China's political economy, China's bureaucracy, development strategies in emerging markets, complex systems, conditions for effective adaptation Dr. -

China's Healthcare System: Addressing Capacity Shortfalls
March 31, 2021 China’s Healthcare System: Addressing Capacity Shortfalls before and after COVID-19 Leyton Nelson, Policy Analyst, Economics and Trade Acknowledgements: Virgil Bisio, former Policy Analyst, Economics and Trade, contributed research to this report. Disclaimer: This paper is the product of professional research performed by staff of the U.S.-China Economic and Security Review Commission, and was prepared at the request of the Commission to support its deliberations. Posting of the report to the Commission’s website is intended to promote greater public understanding of the issues addressed by the Commission in its ongoing assessment of U.S.- China economic relations and their implications for U.S. security, as mandated by Public Law 106-398 and Public Law 113-291. However, the public release of this document does not necessarily imply an endorsement by the Commission, any individual Commissioner, or the Commission’s other professional staff, of the views or conclusions expressed in this staff research report. ! Table of Contents Key Findings .............................................................................................................................................................. 1 Introduction ................................................................................................................................................................ 1 Chronic Disease and Demographic Trends Strain China’s Healthcare System ......................................................... 1 As China’s Population -

Expensive Patients, Reinsurance, and the Future of Health Care Reform
Emory Law Journal Volume 69 Issue 6 2020 Expensive Patients, Reinsurance, and the Future of Health Care Reform Govind Persad Follow this and additional works at: https://scholarlycommons.law.emory.edu/elj Recommended Citation Govind Persad, Expensive Patients, Reinsurance, and the Future of Health Care Reform, 69 Emory L. J. 1153 (2020). Available at: https://scholarlycommons.law.emory.edu/elj/vol69/iss6/1 This Article is brought to you for free and open access by the Journals at Emory Law Scholarly Commons. It has been accepted for inclusion in Emory Law Journal by an authorized editor of Emory Law Scholarly Commons. For more information, please contact [email protected]. PERSAD_8.21.20 8/24/2020 11:43 AM EXPENSIVE PATIENTS, REINSURANCE, AND THE FUTURE OF HEALTH CARE REFORM Govind Persad* ABSTRACT In 2017, Americans spent over $3.4 trillion—nearly 18% of gross domestic product—on health care. This spending is unevenly distributed: Almost a quarter is spent on the costliest 1% of patients, and almost half on the costliest 5%. Most of these patients soon return to a lower percentile, but many continue to incur health care costs in the top percentiles year after year. This Article focuses on the challenges that persistently expensive patients present for health law and policy, and how fairly dividing their medical costs among payers illuminates fundamental normative choices about the design and reform of health insurance. In doing so, this Article draws on bioethical and health policy analyses of the fair distribution of medical costs, and examines how legal doctrine shapes health systems’ options for responding to expensive patients. -

The Citizenship Advantage in Psychological Well-Being: an Examination of the Hukou System in China
Demography (2021) 58(1):165–189 Published online: 12 January 2021 DOI 10.1215/00703370-8913024 © 2021 The Authors This is an open ac cess ar ti cle dis trib uted un der the terms of a Creative Commons license (CC BY-NC-ND 4.0). The Citizenship Advantage in Psychological Well-being: An Examination of the Hukou System in China Qian Song and James P. Smith ABSTRACT Given that Chi nese mi grants with rural hukou sta tus are not consid ered full cit i zens in their ur ban des ti na tions, ru ral-ur ban hukou conver sion signifies full cit i zen ship at tain ment in urban China. We assess causal ef fects of three major types of ur ban hukou at tain ment—mer it-, pol i cy-, and fam i ly-based hukou con ver sion—on mi grants’ psy cho log i cal well-be ing in mid dle- and lat er-life. We fur ther ex am ine how hukou mat ters—how pe ri ods and hukou des ti na tions alter the values of spe cific urban hukou and their psy cho log i cal health im pli ca tions for in di vid u als. We use the China Health and Retirement Longitudinal Study (2015 data) and life history data (for 2014) for anal y sis. To as sess the ex tent to which the salmon ef fect contrib utes to es ti ma tion bias for migrants, we compare re sults from a sample with current migrants and one with cur rent and returned migrants. -

Addressing Health Care Market Consolidation and High Prices the Role of the States
HEALTH POLICY CENTER RESEARCH REPORT Addressing Health Care Market Consolidation and High Prices The Role of the States Robert A. Berenson Jaime S. King Katherine L. Gudiksen Roslyn Murray URBAN INSTITUTE UC HASTINGS LAW UC HASTINGS LAW GEORGETOWN UNIVERSITY Adele Shartzer URBAN INSTITUTE January 2020 ABOUT THE URBAN INSTITUTE The nonprofit Urban Institute is a leading research organization dedicated to developing evidence-based insights that improve people’s lives and strengthen communities. For 50 years, Urban has been the trusted source for rigorous analysis of complex social and economic issues; strategic advice to policymakers, philanthropists, and practitioners; and new, promising ideas that expand opportunities for all. Our work inspires effective decisions that advance fairness and enhance the well-being of people and places. Copyright © January 2020. Urban Institute. Permission is granted for reproduction of this file, with attribution to the Urban Institute. Cover image by Tim Meko. Contents Acknowledgments iv Executive Summary v 1. Introduction 1 2. Employee Retirement Income Security’s Act 8 3. State Efforts to Promote Transparency 11 4. State Efforts to Regulate Consolidation and Promote Competition 17 5. State Options for Overseeing and Regulating Prices 44 6. Conclusion 68 Notes 69 References 79 About the Authors 86 Statement of Independence 87 Acknowledgments This report was funded by the Commonwealth Fund. We are grateful to them and to all our funders, who make it possible for Urban to advance its mission. The views expressed are those of the authors and should not be attributed to the Urban Institute or UC Hastings Law, its trustees, or its funders. -
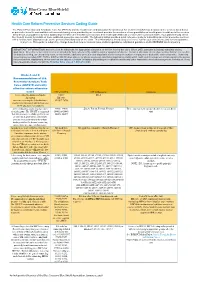
Health Care Reform Preventive Services Coding Guide
Health Care Reform Preventive Services Coding Guide The Patient Protection and Affordable Care Act (PPACA) and the Health Care and Education Reconciliation Act of 2010 (HCERA) has designated the services listed below as preventive benefits and available with no cost-sharing when provided by an in-network provider for members of non-grandfathered health plans. In addition to the services listed below, your patient may have additional preventive care benefits covered under their health plan that may or may not be covered at 100%. Your patient should check their benefit booklet for details on these additional preventive care benefits. The following tables provide a quick reference guide for submitting claims for preventive services with a “well-person” diagnosis code as the primary (first) diagnosis on the claim. This information is intended as a reference tool for your convenience and is not a guarantee of payment. This guide is subject to change based on new or revised laws and/or regulations, additional guidance and/or BCBSNC medical policy. IMPORTANT INFORMATION: Services must be billed with the appropriate diagnosis, at the line level of the claim (Block 24E), pursuant to industry standard coding guidelines. Preventive or screening services are intended for those who currently exhibit no signs or symptoms of disease. Services otherwise deemed preventive that are received in an inpatient setting, an emergency room, or that include additional procedures or diagnostic services may be subject to copayment, deductible and coinsurance. Submitting screening service codes (CPT, HCPCs, ICD-9 or ICD-10) when signs or symptoms are present constitutes inappropriate coding which could result in recoupment of monies paid to the provider for those services. -

The Intergenerational Inequality of Health in China
The Intergenerational Inequality of Health in China Tor Eriksson Department of Economics and Business, Business and Social Sciences, Aarhus University Jay Pan West China School of Public Health, Sichuan University Western China Research Center for Rural Health Development, Sichuan University Xuezheng Qin School of Economics, Peking University Contact information of authors: Tor Eriksson Address: Fuglesangs Allé 4, Building 2632/L111, 8210 Aarhus V, Denmark Telephone: +45-871-64978 Email: [email protected] Jay Pan Address: West China School of Public Health, Sichuan University, Chengdu, China, 610041 Telephone: +86-28-8550-1272 Fax: +86-28-8550-1528 Email: [email protected] Xuezheng Qin (corresponding author) Address: School of Economics, Peking University, Beijing, China, 100871 Telephone: +86-10-6275-7237 Fax: +86-10-6275-4237 Email: [email protected] Acknowledgements: We are grateful to the National Natural Science Foundation of China (Grant No. 71103009), Ministry of Education of China (Grant No. 12JZD036) and the Sino-Danish Center for Education and Research for their financial support. The authors are responsible for all remaining errors. 1 The Intergenerational Inequality of Health in China Tor Eriksson Department of Economics and Business, Business and Social Sciences, Aarhus University Jay Pan West China School of Public Health, Sichuan University Western China Research Center for Rural Health Development, Sichuan University Xuezheng Qin School of Economics, Peking University Abstract: This paper estimates the intergenerational health transmission in China using the 1991-2009 China Health and Nutrition Survey (CHNS) data. Three decades of persistent economic growth in China has been accompanied by high income inequality, which may in turn be caused by the inequality of opportunity in education and health. -

Big Bang Health Care Reform — Does It Work?: the Case of Britain’S 1991 National Health Service Reforms
Big Bang Health Care Reform — Does It Work?: The Case of Britain’s 1991 National Health Service Reforms RUDOLF KLEIN University o f Bath, England ealth care reform has b e e n o n e of the worldwide epidemics of the 1990s. But among the many coun tries that have either attempted or contemplated the reform H of their health care systems (Hurst 1992; Organization for Economic Co-operation and Development 1994) Britain stands out from the rest. The reforms of the National Health Service (NHS) introduced in 1991 by Mrs. Thatcher’s Conservative government were driven by the much the same set of concerns and ideas that shaped the international vocabu lary of debate. In particular, they reflected the widely held belief that the best way of improving efficiency was to change the incentives to pro viders and that some form of marketlike competition was the best tool for achieving this aim (Saltman and van Otter 1992). In all these re spects, there was nothing all that special about Britain. What makes the British case special, and worthy of further study, however, is the ambi tious scope of the reforms and the relentless determination with which Mrs. Thatcher’s government implemented them. In the United States, the Clinton reforms plan foundered on the rocks of congressional oppo sition; in Sweden, a succession of local experiments and committees of inquiry failed to create a national consensus about the direction of health care policy; in the Netherlands, an ambitious plan of reform was The Milbank Quarterly, Vol. 73, No. 3, 1995 © 1995 Milbank Memorial Fund. -

China's Capacity to Manage Infectious Diseases
China’s Capacity to Manage Infectious Diseases CENTER FOR STRATEGIC & Global Implications CSIS INTERNATIONAL STUDIES A Report of the CSIS Freeman Chair in China Studies 1800 K Street | Washington, DC 20006 PROJECT DIRECTOR Tel: (202) 887-0200 | Fax: (202) 775-3199 Charles W. Freeman III E-mail: [email protected] | Web: www.csis.org PROJECT EDITOR Xiaoqing Lu March 2009 ISBN 978-0-89206-580-6 CENTER FOR STRATEGIC & Ë|xHSKITCy065806zv*:+:!:+:! CSIS INTERNATIONAL STUDIES China’s Capacity to Manage Infectious Diseases Global Implications A Report of the CSIS Freeman Chair in China Studies PROJECT DIRECTOR Charles W. Freeman III PROJECT EDITOR Xiaoqing Lu March 2009 About CSIS In an era of ever-changing global opportunities and challenges, the Center for Strategic and Inter- national Studies (CSIS) provides strategic insights and practical policy solutions to decisionmak- ers. CSIS conducts research and analysis and develops policy initiatives that look into the future and anticipate change. Founded by David M. Abshire and Admiral Arleigh Burke at the height of the Cold War, CSIS was dedicated to the simple but urgent goal of finding ways for America to survive as a nation and prosper as a people. Since 1962, CSIS has grown to become one of the world’s preeminent public policy institutions. Today, CSIS is a bipartisan, nonprofit organization headquartered in Washington, D.C. More than 220 full-time staff and a large network of affiliated scholars focus their expertise on defense and security; on the world’s regions and the unique challenges inherent to them; and on the issues that know no boundary in an increasingly connected world. -

Rockefeller Philanthropy and Maternal/Infant Health in the Republic of China
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by IssueLab Rockefeller Philanthropy and Maternal/Infant Health in the Republic of China By Joshua Hubbard University of Michigan [email protected] Note: This research report is presented here with the author’s permission, but should not be cited or quoted without the author’s consent. Rockefeller Archive Center Research Reports Online is an ongoing publication of the Rockefeller Archive Center (RAC) under the general direction of James Allen Smith, Vice President of the RAC and Director of Research and Education. Research Reports Online is intended to foster the network of scholarship in the history of philanthropy and to highlight the diverse range of materials and subjects covered in the collections at the RAC. These reports are drawn from essays submitted by researchers who have visited the Archive Center, most of whom have received grants-in-aid from the Archive Center to support their research. The ideas and opinions expressed in this report are those of the author and not of the Rockefeller Archive Center. The Rockefeller-founded China Medical Board and the Rockefeller Foundation’s International Health Commission/Board/Division (hereafter, IHC/B/D) maintained operations in China from the early to mid-twentieth century. These efforts developed in close relation to Rockefeller-funded endeavors elsewhere in the world, though they soon adopted emphases and methods to address the particularities of the Chinese case. Although initially hindered by a highly volatile and violent political environment, by 1930, these Rockefeller-affiliated health philanthropies forged an enduring partnership with the Kuomintang (KMT or Chinese Nationalist Party)-led government founded in 1927. -

LOCATION of CONFERENCE HALL Robertson Hall, Princeton, NJ 08540
LOCATION OF CONFERENCE HALL Robertson Hall, Princeton, NJ 08540 Page 1 of 19 LOCATION OF MEETING VENUES Page 2 of 19 CONFERENCE COMMITTEE President Xiaogang Wu (Hong Kong University of Science and Technology, HKSAR) Co-sponsor Yu Xie (Princeton University, USA) Members Hua-Yu Sebastian Cherng (New York University, USA) Qiang Fu (The University of British Columbia, CANADA) Reza Hasmath (University of Alberta, CANADA) Anning Hu (Fudan University, Mainland CHINA) Li-Chung Hu (National Chengchi University, TAIWAN) Yingchun Ji (Shanghai University, MAINLAND CHINA) Yingyi Ma (Syracuse University, USA) Lijun Song (Vanderbilt University, USA) Jun Xu (Ball State University, USA) Wei-hsin Yu (University of Maryland, USA) Amy Tsang (Harvard University, USA, Student representative) CONFERENCE SECRETARIAT Duoduo Xu (Hong Kong University of Science and Technology, HKSAR) Phillip Rush (Princeton University, USA) Shaoping Echo She (Hong Kong University of Science and Technology, HKSAR) Page 3 of 19 ORGANIZERS Page 4 of 19 PROGRAMME OUTLINE Friday August 10th 2018 Time Details Venue Bernstein 8:30 – 9:30 Registration and Reception Gallery Opening Speech 9:30 – 10:00 Bowl 016 by Prof. Yu Xie & Prof. Xiaogang Wu Bernstein 10:00 – 10:30 Coffee Break and Group Photo Taking Gallery Parallel Sessions 1.1 Big Data and Deep Learning Bowl 016 1.2 Education and Schooling Bowl 001 10:30 – 12:00 1.3 Gender Norms and Attitudes Bowl 002 (90 min’) 1.4 Migrants and Immigrants Rm. 005 1.5 Family and Domestic Labor Rm. 023 1.6 Governance and Civil Society Rm. 029 1.7 Hospitals, Patients and Medicine Rm. 035 Bernstein 12:00 – 13:20 Lunch Gallery Parallel Sessions 2.1 Marriage and Assortative Mating Bowl 016 2.2 Gender Inequality Bowl 001 13:20 – 14:50 2.3 Intergenerational Transfer and Relation Bowl 002 (90 min’) 2.4 Mental Health and Subjective Well-being Rm. -

The Primary Health-Care System in China
Review The primary health-care system in China Xi Li*, Jiapeng Lu*, Shuang Hu, KK Cheng, Jan De Maeseneer, Qingyue Meng, Elias Mossialos, Dong Roman Xu, Winnie Yip, Hongzhao Zhang, Harlan M Krumholz†, Lixin Jiang†, Shengshou Hu† Lancet 2017; 390: 2584–94 China has made remarkable progress in strengthening its primary health-care system. Nevertheless, the system still *Joint first authors faces challenges in structural characteristics, incentives and policies, and quality of care, all of which diminish its †Joint senior authors preparedness to care for a fifth of the world’s population, which is ageing and which has a growing prevalence of chronic National Clinical Research non-communicable disease. These challenges include inadequate education and qualifications of its workforce, ageing Center of Cardiovascular and turnover of village doctors, fragmented health information technology systems, a paucity of digital data on everyday Diseases, State Key Laboratory clinical practice, financial subsidies and incentives that do not encourage cost savings and good performance, insurance of Cardiovascular Disease, Fuwai Hospital, National policies that hamper the efficiency of care delivery, an insufficient quality measurement and improvement system, and Center for Cardiovascular poor performance in the control of risk factors (such as hypertension and diabetes). As China deepens its health-care Diseases, Chinese Academy reform, it has the opportunity to build an integrated, cooperative primary health-care system, generating knowledge from of Medical Sciences and practice that can support improvements, and bolstered by evidence-based performance indicators and incentives. Peking Union Medical College, Beijing, China (X Li PhD, 1,13 J Lu PhD, Shu Hu PhD, Introduction 2015.