Te Arawa Species Report Koaro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Habitat for Canterbury Freshwater Fish, Kōura/Kēkēwai and Kākahi
CRITICAL HABITAT FOR CANTERBURY FRESHWATER FISH, KŌURA/KĒKĒWAI AND KĀKAHI REPORT PREPARED FOR CANTERBURY REGIONAL COUNCIL BY RICHARD ALLIBONE WATERWAYS CONSULTING REPORT NUMBER: 55-2018 AND DUNCAN GRAY CANTERBURY REGIONAL COUNCIL DATE: DECEMBER 2018 EXECUTIVE SUMMARY Aquatic habitat in Canterbury supports a range of native freshwater fish and the mega macroinvertebrates kōura/kēkēwai (crayfish) and kākahi (mussel). Loss of habitat, barriers to fish passage, water quality and water quantity issues present management challenges when we seek to protect this freshwater fauna while providing for human use. Water plans in Canterbury are intended to set rules for the use of water, the quality of water in aquatic systems and activities that occur within and adjacent to aquatic areas. To inform the planning and resource consent processes, information on the distribution of species and their critical habitat requirements can be used to provide for their protection. This report assesses the conservation status and distributions of indigenous freshwater fish, kēkēwai and kākahi in the Canterbury region. The report identifies the geographic distribution of these species and provides information on the critical habitat requirements of these species and/or populations. Water Ways Consulting Ltd Critical habitats for Canterbury aquatic fauna Table of Contents 1 Introduction ......................................................................................................................................... 1 2 Methods .............................................................................................................................................. -

A Global Assessment of Parasite Diversity in Galaxiid Fishes
diversity Article A Global Assessment of Parasite Diversity in Galaxiid Fishes Rachel A. Paterson 1,*, Gustavo P. Viozzi 2, Carlos A. Rauque 2, Verónica R. Flores 2 and Robert Poulin 3 1 The Norwegian Institute for Nature Research, P.O. Box 5685, Torgarden, 7485 Trondheim, Norway 2 Laboratorio de Parasitología, INIBIOMA, CONICET—Universidad Nacional del Comahue, Quintral 1250, San Carlos de Bariloche 8400, Argentina; [email protected] (G.P.V.); [email protected] (C.A.R.); veronicaroxanafl[email protected] (V.R.F.) 3 Department of Zoology, University of Otago, P.O. Box 56, Dunedin 9054, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +47-481-37-867 Abstract: Free-living species often receive greater conservation attention than the parasites they support, with parasite conservation often being hindered by a lack of parasite biodiversity knowl- edge. This study aimed to determine the current state of knowledge regarding parasites of the Southern Hemisphere freshwater fish family Galaxiidae, in order to identify knowledge gaps to focus future research attention. Specifically, we assessed how galaxiid–parasite knowledge differs among geographic regions in relation to research effort (i.e., number of studies or fish individuals examined, extent of tissue examination, taxonomic resolution), in addition to ecological traits known to influ- ence parasite richness. To date, ~50% of galaxiid species have been examined for parasites, though the majority of studies have focused on single parasite taxa rather than assessing the full diversity of macro- and microparasites. The highest number of parasites were observed from Argentinean galaxiids, and studies in all geographic regions were biased towards the highly abundant and most widely distributed galaxiid species, Galaxias maculatus. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

Evidence of Interactive Segregation Between Introduced Trout and Native Fishes in Northern Patagonian Rivers, Chile
Transactions of the American Fisheries Society 138:839–845, 2009 [Note] Ó Copyright by the American Fisheries Society 2009 DOI: 10.1577/T08-134.1 Evidence of Interactive Segregation between Introduced Trout and Native Fishes in Northern Patagonian Rivers, Chile BROOKE E. PENALUNA* Department of Fisheries and Wildlife, Oregon State University, 3200 Jefferson Way, Corvallis, Oregon 97331, USA IVAN ARISMENDI Nu´cleo Milenio FORECOS, and Escuela de Graduados, Facultad de Ciencias Forestales, Universidad Austral de Chile, Casilla #567, Valdivia, Chile DORIS SOTO Nu´cleo Milenio FORECOS, Universidad Austral de Chile, Casilla #567, Valdivia, Chile; and Food and Agriculture Organization of the United Nations, Fisheries Department, Inland Water Resources and Aquaculture Service, Viale delle Terme di Caracalla, 00100 Rome, Italy Abstract.—Introduced rainbow trout Oncorhynchus mykiss recreational fishing and early practices of aquaculture and brown trout Salmo trutta fario are the most abundant (Basulto 2003). It was thought that these areas in the fishes in the northern Chilean Patagonia, and their effect on Southern Hemisphere were suitable for and would benefit native fishes is not well known. We tested for interactive from the addition of trout (Campos 1970; Basulto 2003). segregation between trout and native fishes by using a before– Since their introduction, trout have formed naturalized after, control–impact design in which we deliberately reduced the density of trout and observed the response of the native populations and have become the most abundant fish fishes in their mesohabitat use (pool, run, riffle). Three native species, accounting for over 95% of the total biomass in fish species, Brachygalaxias bullocki, Galaxias maculatus rivers of the Chilean Patagonia (Soto et al. -

Mokoia Intermediate School – Te Kura Takawaenga O Mokoia
MMokookioai aIn Itnetremrmedeidaitaete Te TKeu Krau rTaa kTaawkaaewnagean gOa MOo Mkooikaoia PRPORSOPSEPCETCUTSU 2S0 2 0 We aWree aLrime iLtilmesitsl!e s sW! e aWree aPraes sPiaosnsaitoen!a t We!e aWree aMroek Moioak Noioa. 1N! o.1! Meet the ‘A’ Team Chris Bashford Toni Bocock Shannon Brake Ashleen Fahy Jacqui French Jess Gibbs Arihi Harvey Rhys Hohepa Glen Law Tracey Low Doug Maguire Kathryn McMurdo Taimona Panapa Leanne Stewart Liana Te Hau Gail Forge Hori Hapi Lisa Te Whare Deana Turner Te Aroha Wihapi Resource Manager Kaiarahi i te Reo Marianne Hodge Moana Hunter Ellen Leach Heather Lind Katrina Weren Learning Support Learning Support Learning Support Learning Support Learning Support Janet Du Fall Sue Rasdall Annemarie Hyde Jackie Jones Rawiri Wihapi Executive Officer School Secretary DP Curriculum DP Pastoral Principal 2 Principal’s Message Welcome to Mokoia Intermediate School – Te Kura Takawaenga o Mokoia Tena Koutou Whānau Ma My name is Rawiri Wihapi and I am delighted to welcome you be at Mokoia Intermediate. I am both honoured and excited to be the Principal of a school which supports change, builds whakawhanaungatanga (relationships), cares for wellbeing (hauora) and is developing a culture of learning. I am married with two adult children. I am of Tainui and Te Arawa descent. I have been a principal for 16 years and a teacher for the past 27 years. I have a passion for teaching, learning and education. I do my best to support, encourage and be a voice for our Mokoia community, especially our tamariki. I believe we should strive to do our personal best to achieve the highest possible standards of learning in all endeavours that life has to offer. -

BEFORE the COMMISSIONERS on BEHALF of the OTAGO REGIONAL COUNCIL Consent No. RM16.093.01 BETWEEN CRIFFEL WATER LIMITED Applic
BEFORE THE COMMISSIONERS ON BEHALF OF THE OTAGO REGIONAL COUNCIL Consent No. RM16.093.01 BETWEEN CRIFFEL WATER LIMITED Applicant AND OTAGO REGIONAL COUNCIL Consent Authority EVIDENCE OF RICHARD MARK ALLIBONE ____________________________________________________________ GALLAWAY COOK ALLAN LAWYERS DUNEDIN Solicitor to contact: Bridget Irving P O Box 143, Dunedin 9054 Ph: (03) 477 7312 Fax: (03) 477 5564 Email: [email protected] BI-308132-1-352-V4 1 EVIDENCE OF RICHARD MARK ALLIBONE Introduction 1. My name is Richard Mark Allibone. 2. I am the Director and Principal Ecologist of Water Ways Consulting Limited. I hold the following tertiary qualifications; a BSc (Zoology and Geology), an MSc (Zoology) and PhD (Zoology), all from the University of Otago. My research has centred on New Zealand’s native fish with a focus on the New Zealand galaxiids, their taxonomy, life history and threats to these species. 3. I specialise in freshwater ecological research and management for native freshwater fish. I have been a researching native fish for over thirty years. Initially my research between 1990 and 2001 was conducted as a post-graduate student and then as a freshwater fisheries specialist for the Department of Conservation, a Post Doctoral Fellow and fisheries scientist at NIWA, and a Species Protection Officer in the Department of Conservation’s Biodiversity Recovery Unit. During 2002-2004 I was the National Services Manager at the QEII National Trust. Since 2004 I have worked as a consultant; firstly for Kingett Mitchell Limited, then Golder Associates (NZ) Ltd. In November 2014 I formed the company Water Ways Consulting Limited where I am a director and the principal ecologist. -

History of Fishes - Structural Patterns and Trends in Diversification
History of fishes - Structural Patterns and Trends in Diversification AGNATHANS = Jawless • Class – Pteraspidomorphi • Class – Myxini?? (living) • Class – Cephalaspidomorphi – Osteostraci – Anaspidiformes – Petromyzontiformes (living) Major Groups of Agnathans • 1. Osteostracida 2. Anaspida 3. Pteraspidomorphida • Hagfish and Lamprey = traditionally together in cyclostomata Jaws = GNATHOSTOMES • Gnathostomes: the jawed fishes -good evidence for gnathostome monophyly. • 4 major groups of jawed vertebrates: Extinct Acanthodii and Placodermi (know) Living Chondrichthyes and Osteichthyes • Living Chondrichthyans - usually divided into Selachii or Elasmobranchi (sharks and rays) and Holocephali (chimeroids). • • Living Osteichthyans commonly regarded as forming two major groups ‑ – Actinopterygii – Ray finned fish – Sarcopterygii (coelacanths, lungfish, Tetrapods). • SARCOPTERYGII = Coelacanths + (Dipnoi = Lung-fish) + Rhipidistian (Osteolepimorphi) = Tetrapod Ancestors (Eusthenopteron) Close to tetrapods Lungfish - Dipnoi • Three genera, Africa+Australian+South American ACTINOPTERYGII Bichirs – Cladistia = POLYPTERIFORMES Notable exception = Cladistia – Polypterus (bichirs) - Represented by 10 FW species - tropical Africa and one species - Erpetoichthys calabaricus – reedfish. Highly aberrant Cladistia - numerous uniquely derived features – long, independent evolution: – Strange dorsal finlets, Series spiracular ossicles, Peculiar urohyal bone and parasphenoid • But retain # primitive Actinopterygian features = heavy ganoid scales (external -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -

SWFSC Archive
Stomiiformes Chapter 4 Order Stomiiformes Number of suborders (2) Gonostomatoidei; Phosichthyoidei (= Photichthyoidei, Stomioidei). Stomiiform monophyly was demonstrated by Fink and Weitzman (1982); relationships within the order are not settled, e.g., Harold and Weitzman (1996). Number of families 4 (or 5: Harold [1998] suggested that Diplophos, Manducus, and Triplophos do not belong in Gonostomatidae, and Nelson [2006] provi sionally placed them in a separate family, Diplophidae). Number of genera 53 Number of species approx. 391 GENERAL LIFE HISTORY REF Distribution All oceans. Relative abundance Rare to very abundant, depending on taxon. Adult habitat Small to medium size (to ca. 10-40 cm) inhabitants of epi-, meso-, and bathypelagic zones, some are vertical migrators. EARLY LIFE HISTORY Mode of reproduction All species known or assumed to be oviparous with planktonic eggs and larvae. Knowledge of ELH Eggs known for 9 genera, larvae known for 38 genera. ELH Characters: Eggs: spherical, ca. 0.6-3.6 mm in diameter, commonly with double membrane, yolk segmented, with 0-1 oil globule ca. 0.1-0.4 mm in di ameter, perivitelline space narrow to wide. Larvae: slender and elongate initially but some become deep-bodied (primarily some sternoptychids and stomiids); preanal length ranges from approx. 30%BL to > 70% BL, most commonly > about half BL, some taxa with trailing gut that can be > 100% BL; eyes strongly ellipti cal to round, most commonly elliptical, stalked in some species; spines lacking on head and pectoral girdle except in some sternoptychids; myo- meres range from 29-164, most commonly approx. mid-30's to mid- 60's; photophores form during postflexion and/or transformation stage; pigmentation absent to heavy, most commonly light. -

Overview of the Impacts of Introduced Salmonids on Australian Native Fauna
OVERVIEW OF THE IMPACTS OF INTRODUCED SALMONIDS ON AUSTRALIAN NATIVE FAUNA by P. L. Cadwallader prepared for the Australian Nature Conservation Agency 1996 ~~ AUSTRALIA,,) Overview of the Impacts of Introduced Salmonids on Australian Native Fauna by P L Cadwallader The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the Commonwealth Government, the Minister for the Environment or the Director of National Parks and Wildlife. ISBN 0 642 21380 1 Published May 1996 © Copyright The Director of National Parks and Wildlife Australian Nature Conservation Agency GPO Box 636 Canberra ACT 2601 Design and art production by BPD Graphic Associates, Canberra Cover illustration by Karina Hansen McInnes CONTENTS FOREWORD 1 SUMMARY 2 ACKNOWLEDGMENTS 3 1. INTRODUCTION 5 2. SPECIES OF SALMONIDAE IN AUSTRALIA 7 2.1 Brown trout 7 2.2 Rainbow trout 8 2.3 Brook trout 9 2.4 Atlantic salmon 9 2.5 Chinook salmon 10 2.6 Summary of present status of salmonids in Australia 11 3. REVIEW OF STUDIES ON THE IMPACTS OF SALMONIDS 13 3.1 Studies on or relating to distributions of salmonids and native fish 13 Grey (1929) Whitley (1935) Williams (1964) Fish (1966) Frankenberg (1966, 1969) Renowden (1968) Andrews (1976) Knott et at. (1976) Cadwallader (1979) Jackson and Williams (1980) Jackson and Davies (1983) Koehn (1986) Jones et al. (1990) Lintermans and Rutzou (1990) Minns (1990) Sanger and F ulton (1991) Sloane and French (1991) Shirley (1991) Townsend and Growl (1991) Hamr (1992) Ault and White (1994) McIntosh et al. (1994) Other Observations and Comments 3.2 Studies Undertaken During the Invasion of New Areas by Salmonids 21 Tilzey (1976) Raadik (1993) Gloss and Lake (in prep) 3.3 Experimental Introduction study 23 Fletcher (1978) 3.4 Feeding Studies, Including Analysis of Dietary Overlap and Competition, and Predation 25 Introductory Comments Morrissy (1967) Cadwallader (1975) Jackson (1978) Cadwallader and Eden (1981,_ 1982) Sagar and Eldon (1983) Glova (1990) Glova and Sagar (1991) Kusabs and Swales (1991) Crowl et at. -
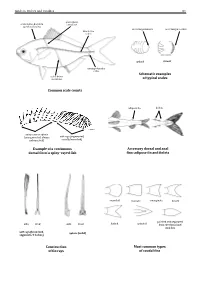
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery.