Field Observations on Courtship and Spawning Behavior O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AGE and GROWTH of the GIANT SEA BASS, STEREOLEPIS GIGAS Calcofi Rep., Vol
HAWK AND ALLEN: AGE AND GROWTH OF THE GIANT SEA BASS, STEREOLEPIS GIGAS CalCOFI Rep., Vol. 55, 2014 AGE AND GROWTH OF THE GIANT SEA BASS, STEREOLEPIS GIGAS HOLLY A. HAWK LARRY G. ALLEN Department of Biology Department of Biology California State University California State University Northridge, CA 91330-8303 Northridge, CA 91330-8303 ph: (818) 677-3356 [email protected] ABSTRACT bass stocks to the point that a moratorium was declared The giant sea bass, Stereolepis gigas, is the largest bony in 1982. Although this species cannot be targeted, com- fish that inhabits California shallow rocky reef com- mercial vessels are now allowed to retain and sell one munities and is listed by IUCN as a critically endan- individual per trip as incidental catch. Giant sea bass gered species, yet little is known about its life history. To caught in Mexican waters by recreational anglers are address questions of growth and longevity, 64 samples allowed to be landed and sold in California markets; were obtained through collaborative efforts with com- however the limit is two fish per trip per angler. There mercial fish markets and scientific gillnetting. Sagittae are no commercial or recreational restrictions on giant (otoliths) were cross-sectioned and analyzed with digi- sea bass in Mexico today (Baldwin and Keiser 2008). tal microscopy. Age estimates indicate that S. gigas is a In 1994, gillnetting was banned within state waters long-lived species attaining at least 76 years of age. Over (three miles off mainland) and one mile from the Chan- 90% of the variation between age (years) and standard nel Islands in southern California. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Wreckfish ITQ Review
Review of the Wreckfish Individual Transferable Quota Program of the South Atlantic Fishery Management Council August 2019 A publication of the South Atlantic Fishery Management Council pursuant to National Oceanic and Atmospheric Administration Award Number FNA10NMF4410012 Table of Contents Abbreviations ...............................................................................................................................................3 List of Tables ...............................................................................................................................................4 List of Figures ..............................................................................................................................................5 Executive Summary .....................................................................................................................................6 1 Introduction and Background ..............................................................................................................7 1.1 Legal requirements for the review ............................................................................................... 7 1.2 Pre-ITQ management ................................................................................................................... 9 1.3 ITQ program description ............................................................................................................ 10 1.3.1 ITQ Goals and Objectives .................................................................................................. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Biology, Stock Status and Management Summaries for Selected Fish Species in South-Western Australia
Fisheries Research Report No. 242, 2013 Biology, stock status and management summaries for selected fish species in south-western Australia Claire B. Smallwood, S. Alex Hesp and Lynnath E. Beckley Fisheries Research Division Western Australian Fisheries and Marine Research Laboratories PO Box 20 NORTH BEACH, Western Australia 6920 Correct citation: Smallwood, C. B.; Hesp, S. A.; and Beckley, L. E. 2013. Biology, stock status and management summaries for selected fish species in south-western Australia. Fisheries Research Report No. 242. Department of Fisheries, Western Australia. 180pp. Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Fisheries Western Australia. While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Department of Fisheries Western Australia does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. Fish illustrations Illustrations © R. Swainston / www.anima.net.au We dedicate this guide to the memory of our friend and colleague, Ben Chuwen Department of Fisheries 3rd floor SGIO Atrium 168 – 170 St Georges Terrace PERTH WA 6000 Telephone: (08) 9482 7333 Facsimile: (08) 9482 7389 Website: www.fish.wa.gov.au ABN: 55 689 794 771 Published by Department of Fisheries, Perth, Western Australia. Fisheries Research Report No. 242, March 2013. ISSN: 1035 - 4549 ISBN: 978-1-921845-56-7 ii Fisheries Research Report No.242, 2013 Contents ACKNOWLEDGEMENTS ............................................................................................... -

Fishery Science: the Unique Contributions of Early Life Stages
Fishery Science: The Unique Contributions of Early Life Stages Lee A. Fuiman Robert G. Werner Blackwell Science 00 03/05/2002 08:37 Page i Fishery Science 00 03/05/2002 08:37 Page ii We dedicate this book to our good friend John Blaxter, the gentleman scientist. His scientific excellence and creativity as well as his personal charm and good humor have made permanent impressions on both of us. John’s scientific contributions permeate this book, which we hope will carry his legacy to many future generations of fishery scientists. 00 03/05/2002 08:37 Page iii Fishery Science The Unique Contributions of Early Life Stages Edited by Lee A. Fuiman Department of Marine Science, University of Texas at Austin, Marine Science Institute, Port Aransas, Texas, USA and Robert G. Werner College of Environmental Science and Forestry, State University of New York, Syracuse, New York, USA 00 03/05/2002 08:37 Page iv © 2002 by Blackwell Science Ltd, First published 2002 by Blackwell Science Ltd a Blackwell Publishing Company Editorial Offices: Library of Congress Osney Mead, Oxford OX2 0EL, UK Cataloging-in-Publication Data Tel: +44 (0)1865 206206 is available Blackwell Science, Inc., 350 Main Street, Malden, MA 02148-5018, USA ISBN 0-632-05661-4 Tel: +1 781 388 8250 Iowa State Press, a Blackwell Publishing A catalogue record for this title is available from Company, 2121 State Avenue, Ames, Iowa the British Library 50014-8300, USA Tel: +1 515 292 0140 Set in Times by Gray Publishing, Tunbridge Blackwell Science Asia Pty, 54 University Street, Wells, Kent Carlton, Victoria 3053, Australia Printed and bound in Great Britain by Tel: +61 (0)3 9347 0300 MPG Books, Bodmin, Cornwall Blackwell Wissenschafts Verlag, Kurfürstendamm 57, 10707 Berlin, Germany Tel: +49 (0)30 32 79 060 For further information on Blackwell Science, visit our website: The right of the Author to be identified as the www.blackwell-science.com Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. -

Sperm Competition and Sex Change: a Comparative Analysis Across Fishes
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2007.00050.x SPERM COMPETITION AND SEX CHANGE: A COMPARATIVE ANALYSIS ACROSS FISHES Philip P. Molloy,1,2,3 Nicholas B. Goodwin,1,4 Isabelle M. Cot ˆ e, ´ 3,5 John D. Reynolds,3,6 Matthew J. G. Gage1,7 1Centre for Ecology, Evolution and Conservation, School of Biological Sciences, University of East Anglia, Norwich, NR4 7TJ, United Kingdom 2E-mail: [email protected] 3Department of Biological Sciences, Simon Fraser University, Burnaby, British Columbia, V5A 1S6, Canada 4E-mail: [email protected] 5E-mail: [email protected] 6E-mail: [email protected] 7E-mail: [email protected] Received October 2, 2006 Accepted October 26, 2006 Current theory to explain the adaptive significance of sex change over gonochorism predicts that female-first sex change could be adaptive when relative reproductive success increases at a faster rate with body size for males than for females. A faster rate of reproductive gain with body size can occur if larger males are more effective in controlling females and excluding competitors from fertilizations. The most simple consequence of this theoretical scenario, based on sexual allocation theory, is that natural breeding sex ratios are expected to be female biased in female-first sex changers, because average male fecundity will exceed that of females. A second prediction is that the intensity of sperm competition is expected to be lower in female-first sex-changing species because larger males should be able to more completely monopolize females and therefore reduce male–male competition during spawning. -

Age and Growth of Dusky Grouper (Epinephelus Marginatus
311 Abstract—We examined the age Age and growth of dusky grouper (Epinephelus and growth of dusky grouper (Epinephelus marginatus) at off- marginatus) (Perciformes: Epinephelidae) shore Carpinteiro Bank (32°16′S; in the southwestern Atlantic, with a size 52°47′W) in the southwestern Atlantic through the analysis of comparison of offshore and littoral habitats growth increments in otoliths. We also examined the hypoth- Mario V. Condini (contact author)1 esis that this offshore habitat Cristiano Q. Albuquerque2 represents a superior site for Alexandre M. Garcia1 fish growth compared with in- shore habitats. Samples con- Email address for contact author: [email protected] sisted of 211 groupers captured by small-scale fisheries between 1 Instituto de Oceanografia 2008 and 2011 and with total Universidade Federal do Rio Grande lengths ranging from 150 to Avenida Itália km 8, Carreiros 1160 mm. Otolith growth incre- 96201-900, Rio Grande ments were deposited once per Rio Grande do Sul, Brazil year, and opaque bands formed 2 Departamento de Oceanografia e Ecologia mostly during the summer, as Universidade Federal do Espírito Santo determined through marginal 29075-900, Vitória increment analysis. Ages ranged Espírito Santo, Brazil from 1 to 40 years, and most fish were aged to be between 2 and 8 years (global mean: 7.4 years, standard deviation 6.9). Von Bertalanffy growth parameters for pooled sexes were L∞=900.9 The dusky grouper (Epinephelus Harmelin-Vivien, 2004), with a de- mm, K=0.129 and t0=−1.45. Fish marginatus) (Epinephelidae) inhab- -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -
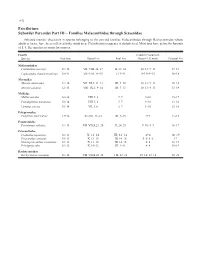
Perciformes Suborder Percoidei Part III – Families Malacanthidae
1152 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid families Malacanthidae through Rachycentridae whose adults or larvae have been collected in the study area. Classification sequence is alphabetical. Most taxa have pelvic fin formula of I, 5. See species accounts for sources. Family Caudal (Procurrent, Species Vertebrae Dorsal Fin Anal Fin Dorsal + Ventral) Pectoral Fin Malacanthidae Caulolatilus microps 11+16 VII–VIII, 24–27 II, 22–24 10–13+9–13 17–18 Lopholatilus chamaeleonticeps 10+14 VII–VIII, 14–15 I, 13–14 9–13+9–13 16–18 Moronidae Morone americana 11+14 VII–XI, I, 11–13 III, 9–10 10–13+9–13 10–18 Morone saxatilis 12+13 VIII–IX, I, 9–14 III, 7–13 10–13+9–13 13–19 Mullidae Mullus auratus 10+14 VIII, I, 8 I, 7 9+10 15–17 Pseudupeneus maculatus 10+14 VIII, I, 8 I, 7 9+10 13–16 Upeneus parvus 10+14 VII, I, 8 I, 7 9+10 15–16 Polyprionidae Polyprion americanus 13+14 XI–XII, 11–12 III, 9–10 9+9 17–18 Pomatomidae Pomatomus saltatrix 11+15 VII–VIII,I,23–28 II, 24–29 9–10+8–9 16–17 Priacanthidae Cookeolus japonicus 10+13 X, 12–14 III, 12–14 4+4 18–19 Priacanthus arenatus 10+13 X, 13–15 III, 14–16 5–6+5–6 17 Heteropriacanthus cruentatus 10+13 X, 13–14 III, 14–15 4+4 18–19 Pristigenys alta 10+13 X, 10–12 III, 9–11 4+4 16–19 Rachycentridae Rachycentron canadum 11+14 VII–VIII,I,26–34 I–II, 22–28 15–16+12–14 20–21 Early Stages of Fishes in the Western North Atlantic Ocean 1153 Perciformes Suborder Percoidei Part III – Families Malacanthidae through Sciaenidae Selected meristic characters in species belonging to the percoid family Sciaenidae whose adults or larvae have been col- lected in the study area. -

Hawk Revised Draft 8 03 13
CALIFORNIA STATE UNIVERSITY, NORTHRIDGE AGE, GROWTH AND GENETIC DIVERSITY OF GIANT SEA BASS, STEREOLEPIS GIGAS A thesis submitted in partial fulfillment of the requirements For the degree of Master of Science in Biology By Holly A. Hawk August 2013 The thesis of Holly Hawk is approved: Dr. Virginia M. Oberholzer Vandergon Date Dr. Cindy S. Malone Date Dr. Michael P. Franklin Date Dr. Larry G. Allen, Chair Date California State University, Northridge ii TABLE OF CONTENTS Signature Page ii Acknowledgements iv List of Tables v List of Figures vi Abstract vii Introduction 1 Methods and Materials 6 Results 11 Discussion 13 Literature Cited 25 Appendix I: Haplotype Summary Table 31 Appendix II: Images of Selected Otoliths 32 iii ACKNOWLEDGMENTS This study would not have been possible without the help and support of many people. Dr. Larry Allen gave me the opportunity to pursue a master’s degree. He has supported me and taught me to grow a thick skin. I would like to thank Dr. Gini Vandergon for agreeing to serve on my committee and for nourishing my love of science education. I also thank her for giving me countless opportunities to educate others. I appreciate the support and guidance of my other committee members, Drs. Michael Franklin and Cindy Malone. Dr. Franklin spent many hours answering many questions. Dr. Malone has not only had invaluable input but has been incredibly supportive and always makes me laugh. Of course, none of this would have been possible without the love and support of my friends and family. I am so incredibly lucky to be surrounded by such wonderful people. -

The Parasite Fauna of the Wreckfish, Polyprion Americanus, in the North Atlantic Ocean; Application to Host Biology and Stock Identification Introduction
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1998 The Parasite Fauna of the Wreckfish, olyprionP americanus, in the North Atlantic Ocean: Application to Host Biology and Stock Identification Colleen Jill Fennessy College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Marine Biology Commons, Oceanography Commons, and the Parasitology Commons Recommended Citation Fennessy, Colleen Jill, "The Parasite Fauna of the Wreckfish, Polyprion americanus, in the North Atlantic Ocean: Application to Host Biology and Stock Identification" (1998). Dissertations, Theses, and Masters Projects. Paper 1539617972. https://dx.doi.org/doi:10.25773/v5-20yb-nh15 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. THE PARASITE FAUNA OF THE WRECKHSH, POLYPRION AMERICANUS, IN THE NORTH ATLANTIC OCEAN: APPLICATION TO HOST BIOLOGY AND STOCK IDENTIFICATION A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary In Partial Fulfillment Of the Requirements for the Degree of Master of Arts by Colleen J. Fennessy 1998 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science Colleen Jill Fennessy Approved, December 1998 olfgrnlg K. Vogelbein lefmey D. Shields Committee Co-Chairman Committee Co-Chairman Eugene M.