Unusual Microbiological Presentations in Polymicrobial Post-Operative Endophthalmitis and Their Clinical Correlations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Simplified Trachoma Grading System, Amended Anthony W Solomon,A Amir B Kello,B Mathieu Bangert,A Sheila K West,C Hugh R Taylor,D Rabebe Tekeraoie & Allen Fosterf
PolicyPolicy & practice & practice The simplified trachoma grading system, amended Anthony W Solomon,a Amir B Kello,b Mathieu Bangert,a Sheila K West,c Hugh R Taylor,d Rabebe Tekeraoie & Allen Fosterf Abstract A simplified grading system for trachoma was published by the World Health Organization (WHO) in 1987. Intended for use by non-specialist personnel working at community level, the system includes five signs, each of which can be present or absent in any eye: (i) trachomatous trichiasis; (ii) corneal opacity; (iii) trachomatous inflammation—follicular; (iv) trachomatous inflammation—intense; and (v) trachomatous scarring. Though neither perfectly sensitive nor perfectly specific for trachoma, these signs have been essential tools for identifying populations that need interventions to eliminate trachoma as a public health problem. In 2018, at WHO’s 4th global scientific meeting on trachoma, the definition of one of the signs, trachomatous trichiasis, was amended to exclude trichiasis that affects only the lower eyelid. This paper presents the amended system, updates its presentation, offers notes on its use and identifies areas of ongoing debate. Introduction (ii) corneal opacity; (iii) trachomatous inflammation—fol- licular; (iv) trachomatous inflammation—intense; and (v) tra- Trachoma is the most important infectious cause of blindness.1 chomatous scarring.19 Trachomatous inflammation—follicular Repeated conjunctival infection2 with particular strains of and trachomatous inflammation—intense are signs of active Chlamydia trachomatis3–5 -

Ophthalmic Management of Facial Nerve Palsy
Eye (2004) 18, 1225–1234 & 2004 Nature Publishing Group All rights reserved 0950-222X/04 $30.00 www.nature.com/eye 1 2 3 Ophthalmic V Lee , Z Currie and JRO Collin REVIEW management of facial nerve palsy Abstract The facial nerve travels with the eighth cranial nerve through the internal auditory canal and The ophthalmologist plays a pivotal role in the through the internal fallopian canal in the evaluation and rehabilitation of patients with petrous temporal bone for the longest facial nerve palsy. It is crucial to recognize and interosseus course of any cranial nerve (30 mm). treat the potentially life-threatening The fibres for the pterygopalatine ganglion underlying causes. The immediate ophthalmic leave at the geniculate ganglion as the greater priority is to ensure adequate corneal superficial petrosal nerve. The nerve to the protection. The medium to long-term stapedius and the chorda tympani (innervation management consists of treatment of epiphora, to the salivary glands) leave prior to the nerve hyperkinetic disorders secondary to aberrant exiting through the stylomastoid foramen as a regeneration and poor cosmesis. Patients purely motor nerve to the muscles of facial should be appropriately referred for general expression.2 Within the substance of the parotid 1 facial re-animation. This review aims to Central Eye Service gland, it divides into the five main Central Middlesex Hospital provide a guide to the management of this branchesFthe temporal, zygomatic, buccal, Acton Lane complex condition. Park Royal mandibular, and cervical branches. Facial nerve Eye (2004) 18, 1225–1234. doi:10.1038/sj.eye.6701383 Acton London, UK lesions above the geniculate ganglion classically Published online 16 April 2004 cause more severe ophthalmic symptoms 2Department of because lacrimal secretion and orbicularis Keywords: gold weight; tarsorrhapy; facial Ophthalmology closure are involved. -

CAUSES, COMPLICATIONS &TREATMENT of A“RED EYE”
CAUSES, COMPLICATIONS & TREATMENT of a “RED EYE” 8 Most cases of “red eye” seen in general practice are likely to be conjunctivitis or a superficial corneal injury, however, red eye can also indicate a serious eye condition such as acute angle glaucoma, iritis, keratitis or scleritis. Features such as significant pain, photophobia, reduced visual acuity and a unilateral presentation are “red flags” that a sight-threatening condition may be present. In the absence of specialised eye examination equipment, such as a slit lamp, General Practitioners must rely on identifying these key features to know which patients require referral to an Ophthalmologist for further assessment. Is it conjunctivitis or is it something more Iritis is also known as anterior uveitis; posterior uveitis is serious? inflammation of the choroid (choroiditis). Complications include glaucoma, cataract and macular oedema. The most likely cause of a red eye in patients who present to 4. Scleritis is inflammation of the sclera. This is a very rare general practice is conjunctivitis. However, red eye can also be presentation, usually associated with autoimmune a feature of a more serious eye condition, in which a delay in disease, e.g. rheumatoid arthritis. treatment due to a missed diagnosis can result in permanent 5. Penetrating eye injury or embedded foreign body; red visual loss. In addition, the inappropriate use of antibacterial eye is not always a feature topical eye preparations contributes to antimicrobial 6. Acid or alkali burn to the eye resistance. The patient history will usually identify a penetrating eye injury Most general practice clinics will not have access to specialised or chemical burn to the eye, but further assessment may be equipment for eye examination, e.g. -
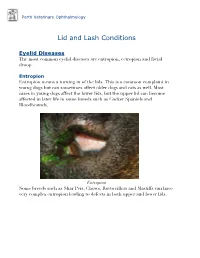
Lid and Lash Conditions
Perth Veterinary Ophthalmology Lid and Lash Conditions Eyelid Diseases The most common eyelid diseases are entropion, ectropion and facial droop. Entropion Entropion means a turning in of the lids. This is a common complaint in young dogs but can sometimes affect older dogs and cats as well. Most cases in young dogs affect the lower lids, but the upper lid can become affected in later life in some breeds such as Cocker Spaniels and Bloodhounds. Entropion Some breeds such as Shar Peis, Chows, Rottweillers and Mastiffs can have very complex entropion leading to defects in both upper and lower lids. A Shar Pei with severe upper and lower lid entropion Entropion is painful and can be potentially blinding. The rolling in of the lid leads to hair coming into contact with the cornea, leading to pain, ulceration and scarring (which can affect vision). In severe cases this can even lead to perforation of the eye. There are many causes of entropion. It can be primary or secondary to other problems affecting the lids (such as ectopic cilia, distichiasis etc. - see below). Some possible causes include the lid being too long, the lid being too tight, instability of the lateral canthus (outer cornea of the eyelids), misdirection of the lateral canthal tendon, brachycephalic anatomy (big eyes and short nose - e.g. Pekingese, Pugs, Shih Tsus, Persian cats etc.), diamond eye defects, loose or too much skin, facial droop etc. Often these cases are referred to a veterinary ophthalmologist for proper assessment and treatment to provide the best outcome. Entropion requires surgical correction. -
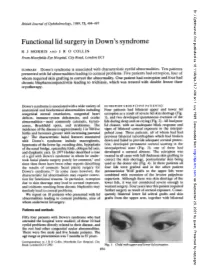
Functional Lid Surgery in Down's Syndrome
Br J Ophthalmol: first published as 10.1136/bjo.73.7.494 on 1 July 1989. Downloaded from British Journal of Ophthalmology, 1989, 73, 494-497 Functional lid surgery in Down's syndrome R J MORRIS AND J R 0 COLLIN From Moorfields Eye Hospital, City Road, London ECI SUMMARY Down's syndrome is associated with characteristic eyelid abnormalities. Ten patients presented with lid abnormalities leading to corneal problems. Five patients had ectropion, four of whom required skin grafting to correct the abnormality. One patient had entropion and four had chronic blepharoconjunctivitis leading to trichiasis, which was treated with double freeze thaw cryotherapy. Down's syndrome is associated with a wide variety of ECTROPION GROUP (FIVE PATIENTS) anatomical and biochemical abnormalities including Four patients had bilateral upper and lower lid congenital mental retardation, congenital heart ectropion as a result of severe lid skin shortage (Fig. defects, immune-system deficiencies, and ocular 1), and two developed spontaneous eversion of the abnormalities-most commonly cataracts, kerato- lids during sleep and on crying (Fig. 2). All had poor conus, Brushfield spots, and strabismus. The lid closure, with an inadequate blink response and incidence of the disease is approximately 1 in 700 live signs of bilateral corneal exposure in the interpal- births and becomes greater with increasing parental pebral zone. Three patients, all of whom had had age.' The characteristic facial features associated previous bilateral tarsorrhaphies which had broken with Down's syndrome include macroglossia, down and failed to provide adequate corneal protec- hypotonia of the lower lip, receding chin, hypoplasia tion, developed permanent corneal scarring in the of the nasal bridge, epicanthic folds, oblique lid axis, interpalpebral zone (Fig. -
Diagnosis and Management of Common Eye Problems
Diagnosis and Management of Common Eye Problems Review of Ocular Anatomy Picture taken from Basic Ophthalmology for Medical Students and Primary Care Residents published by the American Academy of Ophthalmology Diagnosis and Management of Common Eye Problems Fernando Vega, MD Lacrimal system and eye musculature Eyelid anatomy Picture taken from Basic Ophthalmology for Medical Students and Primary Care Residents published by the American Academy of Ophthalmology n Red Eye Disorders: An Anatomical Approach n Lids n Orbit n Lacrimal System n Conjunctivitis n Cornea n Anterior Chamber Fernando Vega, MD 1 Diagnosis and Management of Common Eye Problems Red Eye Disorders: What is not in the scope of Red Eye Possible Causes of a Red Eye n Loss of Vision n Trauma n Vitreous Floaters n Chemicals n Vitreous detatchment n Infection n Retinal detachment n Allergy n Chronic Irritation n Systemic Infections Symptoms can help determine the Symptoms Continued diagnosis Symptom Cause Symptom Cause Itching allergy Deep, intense pain Corneal abrasions, scleritis Scratchiness/ burning lid, conjunctival, corneal Iritis, acute glaucoma, sinusitis disorders, including Photophobia Corneal abrasions, iritis, acute foreign body, trichiasis, glaucoma dry eye Halo Vision corneal edema (acute glaucoma, Localized lid tenderness Hordeolum, Chalazion contact lens overwear) Diagnostic steps to evaluate the patient with Diagnostic steps continued the red eye n Check visual acuity n Estimate depth of anterior chamber n Inspect pattern of redness n Look for irregularities in pupil size or n Detect presence or absence of conjunctival reaction discharge: purulent vs serous n Look for proptosis (protrusion of the globe), n Inspect cornea for opacities or irregularities lid malfunction or limitations of eye n Stain cornea with fluorescein movement Fernando Vega, MD 2 Diagnosis and Management of Common Eye Problems How to interpret findings n Decreased visual acuity suggests a serious ocular disease. -

Trichiasis in Cryotherapy, the Treated Part of the Lid Can Appear Whitish in Colour
Are there any risks or complications? It is likely that the lashes will grow again and scarring may be a problem. Trichiasis In cryotherapy, the treated part of the lid can appear whitish in colour. What is trichiasis? What should I do if it recurs? Trichiasis is an uncomfortable condition in which your eye lashes Please make an appointment with your GP. grow inwards and irritate your eyeball, scratching its surface. What is the cause? The most common cause is chronic inflammation, with scarring of the lower eyelid. This can be caused by infection, skin disease or injury to your eyelid. How is it treated? The eye lashes can be removed using forceps (epilation). This only provides temporary relief as the lashes re-grow. Permanent relief can be obtained by destroying the lash follicle using electrolysis or cryosurgery. Electrolysis is a method in which an electric current is used to kill the cells that produce the hair. Cryosurgery is a newer method where the area is frozen, killing the hair follicles. Is the treatment painful? The lower eyelid is numbed by an injection of local anaesthetic before the electrolysis treatment takes place. This may be slightly painful. Are there any side effects? If you would like this leaflet in large print, braille, audio version Swelling of the eyelid and redness is common for a few days but the or in another language, please contact the General Office on lid should soon return to normal quite quickly. There may be a small notch in the lid contour afterwards. 01872 252690 RCHT 639 © RCHT Design & Publications 2003 Continued overleaf.. -

Keratoconus Inflammatory Associations and Treatment Characteristics KERATOCONUS INFLAMMATORY ASSOCIATIONS and TREATMENT CHARACTERISTICS
Robert Wisse Keratoconus Inflammatory associations and treatment characteristics KERATOCONUS INFLAMMATORY ASSOCIATIONS AND TREATMENT CHARACTERISTICS Thesis, Utrecht University, The Netherlands Copyright © by Robert PL Wisse, 2015 ISBN ISBN 978-94-6233-177-8 Printed by Gildeprint Drukkerijen BV, Enschede Cover Silence et Lumières des Glaces Olieverf op doek, Robert Amrouche, Parijs 2005 Lay out Annelies Wisse, Amsterdam, www.annelieswisse.nl CORRESPONDENCE R.P.L. Wisse, Ophthalmologist | Corneal Specialist Utrecht Cornea Research Group | Department of Ophthalmology Office E.03.136 PO Box 85500 3508 GA Utrecht, The Netherlands Telephone +31 88 75 51683 Email [email protected] Keratoconus Inflammatory associations and treatment characteristics Over de rol van inflammatie en behandeling in keratoconus (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof.dr. G.J. van der Zwaan, ingevolge het besluit van het college voor promoties in het openbaar te verdedigen op dinsdag 22 december 2015 des middags te 2.30 uur door ROBERT PIETER LEENDERT WISSE geboren op 31 maart 1983 te Delft PROMOTOR Prof.dr. S.M. Imhof CO-PROMOTOR Dr. A. van der Lelij The research described in this thesis was financially supported by the Dr. F.P. Fischer- stichting, the Landelijke Stichting voor Blinden en Slechtzienden, and the Stichting Vrienden van het UMC. Printing of this thesis was kindly financially supported by Zeiss Nederland; Thea Pharma Benelux; Visser Contactlenzen BV; de Hoornvlies Patienten Vereniging; EyeMed BV; Simovision; Synga Medical; Ophtec BV; Eye Wish Opticiens Wisse, Raadhuisstraat Roosendaal Aan Julia en Roosmarijn Contents Chapter 1 9 General introduction and thesis outline Chapter 2 37 Does lamellar surgery for keratoconus experience the popularity it deserves? Acta Ophthalmologica 2014 Chapter 3 55 Partial endothelial trepanation in addition to deep anterior lamellar keratoplasty in keratoconus patients. -

Visual Impairment Age-Related Macular
VISUAL IMPAIRMENT AGE-RELATED MACULAR DEGENERATION Macular degeneration is a medical condition predominantly found in young children in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thickening, atrophy, and in some cases, watering. This can result in loss of side vision, which entails inability to see coarse details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) in the United States today for those under the age of twenty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD). Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina which provides detailed central vision, called fovea) called drusen between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure. Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. -

The Definition and Classification of Dry Eye Disease
DEWS Definition and Classification The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry E y e W ork Shop (2 0 0 7 ) ABSTRACT The aim of the DEWS Definition and Classifica- I. INTRODUCTION tion Subcommittee was to provide a contemporary definition he Definition and Classification Subcommittee of dry eye disease, supported within a comprehensive clas- reviewed previous definitions and classification sification framework. A new definition of dry eye was devel- T schemes for dry eye, as well as the current clinical oped to reflect current understanding of the disease, and the and basic science literature that has increased and clarified committee recommended a three-part classification system. knowledge of the factors that characteriz e and contribute to The first part is etiopathogenic and illustrates the multiple dry eye. Based on its findings, the Subcommittee presents causes of dry eye. The second is mechanistic and shows how herein an updated definition of dry eye and classifications each cause of dry eye may act through a common pathway. based on etiology, mechanisms, and severity of disease. It is stressed that any form of dry eye can interact with and exacerbate other forms of dry eye, as part of a vicious circle. II. GOALS OF THE DEFINITION AND Finally, a scheme is presented, based on the severity of the CLASSIFICATION SUBCOMMITTEE dry eye disease, which is expected to provide a rational basis The goals of the DEWS Definition and Classification for therapy. These guidelines are not intended to override the Subcommittee were to develop a contemporary definition of clinical assessment and judgment of an expert clinician in dry eye disease and to develop a three-part classification of individual cases, but they should prove helpful in the conduct dry eye, based on etiology, mechanisms, and disease stage. -

Trichiasis Surgery for Trachoma Second Edition
Trichiasis surgery for trachoma Second Edition ISBN 978 92 4 154867 0 Trichiasis surgery for trachoma Second Edition Shannath Merbs, MD, PhD, Serge Resniko, MD, PhD, Amir Bedri Kello, MD, MSc, Silvio Mariotti, MD, Gregory Greene, MSPH, Sheila K West, PhD Illustrations Tim Phelps, MS, FAMI ACKNOWLEDGEMENTS S. Bakayoko and E. Gower for some images; M. Burton, B. Gaynor and S. Lewallen for their reviews. WHO Library Cataloguing-in-Publication Data Trichiasis surgery for trachoma. Update from « Final assessment of Trichiasis surgeons » and « Trichiasis surgery for trachoma, the bilamellar tarsal rotation procedure » (WHO/PBL/93.29) 1.Trachoma – prevention and control. 2.Blindness – prevention and control. 3.Trachoma - surgery. 4.Trichiasis - surgery. 5.Eyelid diseases. 6.Eyelids – surgery. 7.Teaching materials. I.World Health Organization. ISBN 978 92 4 154867 0 (NLM classication: WW 215) © World Health Organization 2013, reprinted 2014 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO web site (www.who.int/about/licensing/copyright_form/en/index.html). ¥e designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Cryosurgery in Treatment of Trichiasis
Br J Ophthalmol: first published as 10.1136/bjo.66.5.337 on 1 May 1982. Downloaded from British Journal ofOphthalmology, 1982, 66, 337-339 Cryosurgery in treatment of trichiasis SOLA MAJEKODUNMI From the Ophthalmology Unit, Department of Surgery, Lagos University Teaching Hospital, Lagos, Nigeria SUMMARY Ten patients with trichiasis were treated with cryosurgery by the standard retinal probe with nitrous oxide. A double freeze-thaw method was used. Nine ofthe patients had trachomatous trichiasis and one had conjunctival scarring. In only the latter case were the lashes destroyed, and these grew again after 2 months. Trachomatous trichiasis is frequently associated with entropion. The tarsal plate in such cases is often thickened and distorted, with extensive conjunctival scarring. The success of cryosurgical treatment of trichiasis depends not only on the type of probe used but perhaps also on the aetiology of the disease. To our knowledge no similar trial of this form of treatment oftrichiasis has been carried out in eye centres in Africa south ofthe Sahara. Cryosurgery is simple to perform and of great potential use, particularly in areas where there is a shortage of skilled surgeons to perform the delicate eyelid operations often required in trichiasis. The varied aetiology of trichiasis had been widely This paper reports on 10 patients with trichiasis discussed.' In Nigeria trachoma is the commonest treated with cryosurgery at the Lago University cause of trichiasis, and the latter, often associated Teaching Hospital. The patients were followed up for with entropion, presents a major clinical problem 6 to 9 months. which often results in blindness.