Preferred Binding of Gain-Of-Function Mutant P53 to Bidirectional Promoters with Coordinated Binding of ETS1 and GABPA to Multiple Binding Sites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peripheral T Cells Ets-1 Maintains IL-7 Receptor Expression In
The Journal of Immunology Ets-1 Maintains IL-7 Receptor Expression in Peripheral T Cells Roland Grenningloh,*,† Tzong-Shyuan Tai,* Nicole Frahm,†,‡,1 Tomoyuki C. Hongo,‡ Adam T. Chicoine,‡ Christian Brander,†,‡,x,{ Daniel E. Kaufmann,†,‡,‖ and I-Cheng Ho*,† The expression of CD127, the IL-7–binding subunit of the IL-7 R, is tightly regulated during the development and activation of T cells and is reduced during chronic viral infection. However, the molecular mechanism regulating the dynamic expression of CD127 is still poorly understood. In this study, we report that the transcription factor Ets-1 is required for maintaining the expression of CD127 in murine peripheral T cells. Ets-1 binds to and activates the CD127 promoter, and its absence leads to reduced CD127 expression, attenuated IL-7 signaling, and impaired IL-7–dependent homeostatic proliferation of T cells. The expression of CD127 and Ets-1 is strongly correlated in human T cells. Both CD127 and Ets-1 expression are decreased in CD8+ T cells during HIV infection. In addition, HIV-associated loss of CD127 is only observed in Ets-1low effector memory and central memory but not in Ets-1high naive CD8+ T cells. Taken together, our data identify Ets-1 as a critical regulator of CD127 expression in T cells. The Journal of Immunology, 2011, 186: 969–976. nterleukin-7 signals are required for T cell development, GABPa or another Ets protein is responsible for maintaining maintaining the naive T cell pool, mounting proper primary CD127 expression in peripheral T cells is unknown. I responses, and inducing and maintaining CD4+ and CD8+ Ets-1 (E26 transformation-specific sequence) is the founding T cell memory (1–3). -

Agonists and Knockdown of Estrogen Receptor Β Differentially Affect
Schüler-Toprak et al. BMC Cancer (2016) 16:951 DOI 10.1186/s12885-016-2973-y RESEARCH ARTICLE Open Access Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro Susanne Schüler-Toprak1*, Julia Häring1, Elisabeth C. Inwald1, Christoph Moehle2, Olaf Ortmann1 and Oliver Treeck1 Abstract Background: Estrogen receptor β (ERβ) is expressed in the majority of invasive breast cancer cases, irrespective of their subtype, including triple-negative breast cancer (TNBC). Thus, ERβ might be a potential target for therapy of this challenging cancer type. In this in vitro study, we examined the role of ERβ in invasion of two triple-negative breast cancer cell lines. Methods: MDA-MB-231 and HS578T breast cancer cells were treated with the specific ERβ agonists ERB-041, WAY200070, Liquiritigenin and 3β-Adiol. Knockdown of ERβ expression was performed by means of siRNA transfection. Effects on cellular invasion were assessed in vitro by means of a modified Boyden chamber assay. Transcriptome analyses were performed using Affymetrix Human Gene 1.0 ST microarrays. Pathway and gene network analyses were performed by means of Genomatix and Ingenuity Pathway Analysis software. Results: Invasiveness of MBA-MB-231 and HS578T breast cancer cells decreased after treatment with ERβ agonists ERB-041 and WAY200070. Agonists Liquiritigenin and 3β-Adiol only reduced invasion of MDA-MB-231 cells. Knockdown of ERβ expression increased invasiveness of MDA-MB-231 cells about 3-fold. Transcriptome and pathway analyses revealed that ERβ knockdown led to activation of TGFβ signalling and induced expression of a network of genes with functions in extracellular matrix, tumor cell invasion and vitamin D3 metabolism. -

Accompanies CD8 T Cell Effector Function Global DNA Methylation
Global DNA Methylation Remodeling Accompanies CD8 T Cell Effector Function Christopher D. Scharer, Benjamin G. Barwick, Benjamin A. Youngblood, Rafi Ahmed and Jeremy M. Boss This information is current as of October 1, 2021. J Immunol 2013; 191:3419-3429; Prepublished online 16 August 2013; doi: 10.4049/jimmunol.1301395 http://www.jimmunol.org/content/191/6/3419 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130139 Material 5.DC1 References This article cites 81 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/191/6/3419.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Global DNA Methylation Remodeling Accompanies CD8 T Cell Effector Function Christopher D. Scharer,* Benjamin G. Barwick,* Benjamin A. Youngblood,*,† Rafi Ahmed,*,† and Jeremy M. -

Genomic Interaction Between ER and HMGB2 Identifies DDX18 As A
Oncogene (2015) 34, 3871–3880 © 2015 Macmillan Publishers Limited All rights reserved 0950-9232/15 www.nature.com/onc ORIGINAL ARTICLE Genomic interaction between ER and HMGB2 identifies DDX18 as a novel driver of endocrine resistance in breast cancer cells AM Redmond1,2, C Byrne1, FT Bane1, GD Brown2, P Tibbitts1,KO’Brien1, ADK Hill1, JS Carroll2 and LS Young1 Breast cancer resistance to endocrine therapies such as tamoxifen and aromatase inhibitors is a significant clinical problem. Steroid receptor coactivator-1 (SRC-1), a coregulatory protein of the oestrogen receptor (ER), has previously been shown to have a significant role in the progression of breast cancer. The chromatin protein high mobility group box 2 (HMGB2) was identified as an SRC-1 interacting protein in the endocrine-resistant setting. We investigated the expression of HMGB2 in a cohort of 1068 breast cancer patients and found an association with increased disease-free survival time in patients treated with endocrine therapy. However, it was also verified that HMGB2 expression could be switched on in endocrine-resistant tumours from breast cancer patients. To explore the function of this poorly characterized protein, we performed HMGB2 ChIPseq and found distinct binding patterns between the two contexts. In the resistant setting, the HMGB2, SRC-1 and ER complex are enriched at promoter regions of target genes, with bioinformatic analysis indicating a switch in binding partners between the sensitive and resistant phenotypes. Integration of binding and gene expression data reveals a concise set of target genes of this complex including the RNA helicase DDX18. Modulation of DDX18 directly affects growth of tamoxifen-resistant cells, suggesting that it may be a critical downstream effector of the HMGB2:ER complex. -

ETS1, Nfkb and AP1 Synergistically Transactivate the Human GM ± CSF Promoter
Oncogene (1997) 14, 2845 ± 2855 1997 Stockton Press All rights reserved 0950 ± 9232/97 $12.00 ETS1, NFkB and AP1 synergistically transactivate the human GM ± CSF promoter Ross S Thomas1, Martin J Tymms1, Leigh H McKinlay1, M Frances Shannon2, Arun Seth3 and Ismarl Kola1 1Molecular Genetics and Development Group, Institute of Reproduction and Development, Monash University, Melbourne 3168, Australia; 2Division of Human Immunology, Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Adelaide 5000, Australia; 3Department of Pathology, University of Toronto/Women's College Hospital, Toronto, Ontario, Canada Activation of helper T cells results in coordinate Activating signals ultimately result in cellular prolifera- expression of a number of cytokines involved in tion, and transcriptional induction and secretion of a dierentiation, proliferation and activation of the number of cytokines including IL-2 (interleukin-2), IL-3, haematopoietic system. Granulocyte-macrophage colony IFNg (interferon-gamma) and GM ± CSF (granulocyte- stimulating factor (GM ± CSF) is one such cytokine, macrophage colony-stimulating factor) (Stanley et al., whose increased expression results mostly from increases 1985; Miyajima et al., 1988; Arai et al., 1990). These in transcription. Cis-acting elements with NFkB, AP1 cytokines direct the eector functions of various cell and ETS-like binding motifs have been identi®ed in the types involved in an immune response, including B cells, promoter region of the GM ± CSF gene, and are macrophages, mast cells, eosinophils and neutrophils. important or essential for transcriptional activity follow- GM ± CSF expression in activated T cells is ing T cell activation. ETS1 is a transcription factor of regulated by two mechanisms. -

Id3 Induces an Elk-1- and Caspase-8-Dependent Apoptotic Pathway In
ID3 INDUCES AN ELK-1- AND CASPASE-8-DEPENDENT APOPTOTIC PATHWAY IN SQUAMOUS CARCINOMA CELLS A Dissertation submitted to the Faculty of the Graduate School of Arts and Sciences of Georgetown University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biochemistry and Molecular & Cellular Biology By You-shin Chen, M.S. Washington, DC November 18, 2014 Copyright 2014 by You-shin Chen All Rights Reserved ii ID3 INDUCES AN ELK-1- AND CASPASE-8-DEPENDENT APOPTOTIC PATHWAY IN SQUAMOUS CARCINOMA CELLS You-shin Chen, M.S. Thesis Advisor: Dean S. Rosenthal, Ph.D. ABSTRACT Inhibitors of differentiation/DNA binding (Id) proteins are helix-loop-helix (HLH) transcription factors. The Id protein family (Id1-Id4) mediates tissue homeostasis by regulating cellular processes including differentiation, proliferation, and apoptosis. Previously, we found that Id3 induced apoptosis in immortalized human keratinocytes (Simbulan-Rosenthal et al., 2006), consistent with its role as a tumor suppressor (Richter et al., 2012; Schmitz et al., 2012). To investigate the role of Id3 in malignant SCC cells (A431), a tetracycline-regulated inducible system was used to induce Id3 in cell culture and mouse xenograft models. We found that upon Id3 induction, there was a decrease in cell number under low serum conditions, as well as in soft agar. Microarray, RT-PCR, immunoblot, siRNA, and inhibitor studies revealed that Id3 induced expression of Elk-1, an ETS-domain transcription factor, inducing procaspase-8 expression and activation. Id3 deletion mutants revealed that 80 C-terminal amino acids, including the HLH, are important for Id3-induced apoptosis. -

FOXP1 Acts Through a Negative Feedback Loop to Suppress FOXO-Induced Apoptosis
Cell Death and Differentiation (2013) 20, 1219–1229 & 2013 Macmillan Publishers Limited All rights reserved 1350-9047/13 www.nature.com/cdd FOXP1 acts through a negative feedback loop to suppress FOXO-induced apoptosis R van Boxtel1,5, C Gomez-Puerto1,6, M Mokry2,6, A Eijkelenboom3, KE van der Vos1, EES Nieuwenhuis2, BMT Burgering3, EW-F Lam4 and PJ Coffer*,1,2 Transcriptional activity of Forkhead box transcription factor class O (FOXO) proteins can result in a variety of cellular outcomes depending on cell type and activating stimulus. These transcription factors are negatively regulated by the phosphoinositol 3-kinase (PI3K)–protein kinase B (PKB) signaling pathway, which is thought to have a pivotal role in regulating survival of tumor cells in a variety of cancers. Recently, it has become clear that FOXO proteins can promote resistance to anti-cancer therapeutics, designed to inhibit PI3K–PKB activity, by inducing the expression of proteins that provide feedback at different levels of this pathway. We questioned whether such a feedback mechanism may also exist directly at the level of FOXO-induced transcription. To identify critical modulators of FOXO transcriptional output, we performed gene expression analyses after conditional activation of key components of the PI3K–PKB–FOXO signaling pathway and identified FOXP1 as a direct FOXO transcriptional target. Using chromatin immunoprecipitation followed by next-generation sequencing, we show that FOXP1 binds enhancers that are pre-occupied by FOXO3. By sequencing the transcriptomes of cells in which FOXO is specifically activated in the absence of FOXP1, we demonstrate that FOXP1 can modulate the expression of a specific subset of FOXO target genes, including inhibiting expression of the pro-apoptotic gene BIK. -

Identification of Degs in B Cells of Patients with Common Variable
Identification of DEGs in B cells of patients with common variable immunodeficiency and healthy donors Master Degree Project in Systems Biology Molecular Biotechnology Two years Master 120 ECTS Spring semester 2019 Version 1 Shirin Pour Akaber Email: [email protected] Supervisor: Ola Grimsholm (University of Gothenburg) Email: [email protected] Co-supervisor: Andreas Tilevik (University of Skövde) Emails: [email protected] Examiner: Björn Olsson Email: [email protected] University of Skövde, department of Bioscience 541 28 Skövde Abstract Common variable immunodeficiency (CVID) is a rare primary immune deficiency (1:25000) in which patients have a reduction in antibody production and very low titres in one or more of their Ig isotypes, (IgG, IgA and sometimes IgM). This disease can cause different symptoms such as: bronchiectasis, chronic lung disease and even autoimmunity, polyclonal lymphocytic infiltration, lymphoma and death. The underlying causes of CVID are still largely unknown but studies show that different factors like primary B-cell dysfunctions, defects in T cells and antigen-presenting cells are involved. Quantitative analysis of gene expression is of high importance in understanding the molecular mechanisms underlying this diseases´ genome regulation. Next-generation RNA-seq has enabled researchers to analyse both coding and non-coding regions of RNA, and therefore has made it possible to identify differentially expressed genes in large-scale data, especially in polygenic diseases like CVID. The aim for this study was to identify the differentially expressed genes between CVID patients and healthy donors to identify important genes and molecular mechanisms underlying this diseases´ genome regulation. For this matter, whole genome RNA-seq analysis was performed on RNA isolated from sorted peripheral blood naïve and CD27bright memory B cells from healthy donors (n=7) and CVID patients (n=5). -

Transcriptional Repressor HIC1 Contributes to Suppressive Function of Human Induced Regulatory T Cells
This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Ubaid Ullah, Ullah; Andrabi, Syed Bilal Ahmad; Tripathi, Subhash Kumar; Dirasantha, Obaiah; Kanduri, Kartiek; Rautio, Sini; Gross, Catharina C.; Lehtimäki, Sari; Bala, Kanchan; Tuomisto, Johanna; Bhatia, Urvashi; Chakroborty, Deepankar; Elo, Laura L.; Lähdesmäki, Harri; Wiendl, Heinz; Rasool, Omid; Lahesmaa, Riitta Transcriptional Repressor HIC1 Contributes to Suppressive Function of Human Induced Regulatory T Cells Published in: Cell Reports DOI: 10.1016/j.celrep.2018.01.070 Published: 20/02/2018 Document Version Publisher's PDF, also known as Version of record Published under the following license: CC BY-NC-ND Please cite the original version: Ubaid Ullah, U., Andrabi, S. B. A., Tripathi, S. K., Dirasantha, O., Kanduri, K., Rautio, S., Gross, C. C., Lehtimäki, S., Bala, K., Tuomisto, J., Bhatia, U., Chakroborty, D., Elo, L. L., Lähdesmäki, H., Wiendl, H., Rasool, O., & Lahesmaa, R. (2018). Transcriptional Repressor HIC1 Contributes to Suppressive Function of Human Induced Regulatory T Cells. Cell Reports, 22(8), 2094-2106. https://doi.org/10.1016/j.celrep.2018.01.070 This material is protected by copyright and other intellectual property rights, and duplication or sale of all or part of any of the repository collections is not permitted, except that material may be duplicated by you for your research use or educational purposes in electronic or print form. You must obtain permission for any other use. Electronic or print copies may not be offered, whether for sale or otherwise to anyone who is not an authorised user. -
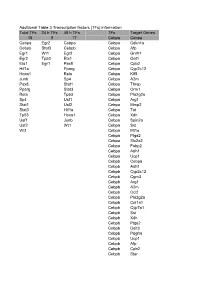
Additional Table 3 Transcription Factors (Tfs) Information Total Tfs
Additional Table 3 Transcription factors (TFs) information Total TFs 24 h TFs 48 h TFs TFs Target Genes 18 5 17 Cebpa Cebpa Cebpa Egr2 Cebpa Cebpa Cdkn1a Cebpb Stat3 Cebpb Cebpa Afp Egr1 Wt1 Egr2 Cebpa Gnrh1 Egr2 Tp53 Ets1 Cebpa Got1 Ets1 Egr1 Pax8 Cebpa Cpb2 Hif1a Pparg Cebpa Cyp2c12 Hoxa1 Rela Cebpa Klf5 Junb Sp4 Cebpa A2m Pax8 Stat1 Cebpa Thrsp Pparg Stat3 Cebpa Orm1 Rela Tp53 Cebpa Pla2g2a Sp4 Usf1 Cebpa Arg1 Stat1 Usf2 Cebpa Mmp2 Stat3 Hif1a Cebpa Tat Tp53 Hoxa1 Cebpa Xdh Usf1 Junb Cebpa Spin2a Usf2 Wt1 Cebpa Sst Wt1 Cebpa Mt1a Cebpa Ptgs2 Cebpa Slc2a2 Cebpa Fabp2 Cebpa Adh1 Cebpa Ucp1 Cebpb Cebpa Cebpb Adh1 Cebpb Cyp2c12 Cebpb Cgm3 Cebpb Arg1 Cebpb A2m Cebpb Ccl2 Cebpb Pla2g2a Cebpb Col1a1 Cebpb Cyp7a1 Cebpb Sst Cebpb Xdh Cebpb Ptgs2 Cebpb Ddit3 Cebpb Pdgfra Cebpb Ucp1 Cebpb Afp Cebpb Cpb2 Cebpb Star Cebpb Prl Cebpb Slc2a2 Egr1 Lhb Egr1 Adrb1 Egr1 Pgy1 Egr1 Th Egr1 Dlk1 Egr1 Chrna7 Egr1 Cntn1 Egr1 Slc9a3 Egr1 Pdgfb Egr1 Aldoc Egr1 Me1 Egr1 Pdgfa Egr1 Ptp4a1 Egr1 Serpine1 Egr1 Atp2a2 Egr1 Lhcgr Egr1 Mmp14 Egr1 Myh6 Egr1 Nudt6 Egr1 F3 Egr1 Id1 Egr2 Aldoc Ets1 Cited2 Ets1 Thy1 Ets1 Lhb Ets1 Runx2 Ets1 Ccnd1 Ets1 Spp1 Ets1 Casp3 Ets1 Prl Ets1 Mmp2 Ets1 Tat Ets1 Ucp1 Ets1 Ccnb1 Ets1 Cdkn1a Ets1 Mmp9 Hif1a Th Hif1a Wt1 Hif1a Vhl Hif1a Nos2 Hif1a Slc2a1 Hif1a Tfrc Hif1a Nppa Hif1a Serpine1 Hif1a Gck Hif1a Adra1a Hif1a Plat Hif1a Hmox1 Hif1a Mt1a Hif1a Pklr Hif1a Cdkn1a Hoxa1 Col1a1 Junb Th Junb Vgf Junb Timp1 Pax8 Wbp2 Pax8 Slc5a5 Pax8 Tg Pparg Hadha Pparg Ucp2 Pparg Aldh3a1 Pparg Nr1d1 Pparg Mapk1 Pparg Atp2a2 Pparg Ptgs2 Pparg Rxrg Pparg -

Crosstalk Between the Glucocorticoid Receptor and Other Transcription Factors: Molecular Aspects Olivier Kassel, Peter Herrlich
Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects Olivier Kassel, Peter Herrlich To cite this version: Olivier Kassel, Peter Herrlich. Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects. Molecular and Cellular Endocrinology, Elsevier, 2007, 275 (1-2), pp.13. 10.1016/j.mce.2007.07.003. hal-00531941 HAL Id: hal-00531941 https://hal.archives-ouvertes.fr/hal-00531941 Submitted on 4 Nov 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects Authors: Olivier Kassel, Peter Herrlich PII: S0303-7207(07)00258-4 DOI: doi:10.1016/j.mce.2007.07.003 Reference: MCE 6679 To appear in: Molecular and Cellular Endocrinology Received date: 17-4-2007 Revised date: 26-6-2007 Accepted date: 3-7-2007 Please cite this article as: Kassel, O., Herrlich, P., Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects, Molecular and Cellular Endocrinology (2007), doi:10.1016/j.mce.2007.07.003 This is a PDF file of an unedited manuscript that has been accepted for publication. -

ZBTB32 Restrains Antibody Responses to Murine Cytomegalovirus Infections, but Not Other Repetitive Challenges Arijita Jash1, You W
www.nature.com/scientificreports OPEN ZBTB32 restrains antibody responses to murine cytomegalovirus infections, but not other repetitive challenges Arijita Jash1, You W. Zhou 2,3, Diana K. Gerardo4, Tyler J. Ripperger4, Bijal A. Parikh 1, Sytse Piersma 2,3, Deepa R. Jamwal5, Pawel R. Kiela5, Adrianus C. M. Boon2,6,7, Wayne M. Yokoyama 2,3, Chyi S. Hsieh2,3 & Deepta Bhattacharya1,4* ZBTB32 is a transcription factor that is highly expressed by a subset of memory B cells and restrains the magnitude and duration of recall responses against hapten-protein conjugates. To defne physiological contexts in which ZBTB32 acts, we assessed responses by Zbtb32−/− mice or bone marrow chimeras against a panel of chronic and acute challenges. Mixed bone marrow chimeras were established in which all B cells were derived from either Zbtb32−/− mice or control littermates. Chronic infection of Zbtb32−/− chimeras with murine cytomegalovirus led to nearly 20-fold higher antigen-specifc IgG2b levels relative to controls by week 9 post-infection, despite similar viral loads. In contrast, IgA responses and specifcities in the intestine, where memory B cells are repeatedly stimulated by commensal bacteria, were similar between Zbtb32−/− mice and control littermates. Finally, an infection and heterologous booster vaccination model revealed no role for ZBTB32 in restraining primary or recall antibody responses against infuenza viruses. Thus, ZBTB32 does not limit recall responses to a number of physiological acute challenges, but does restrict antibody levels during chronic viral infections that periodically engage memory B cells. This restriction might selectively prevent recall responses against chronic infections from progressively overwhelming other antibody specifcities.