FOXP1 Acts Through a Negative Feedback Loop to Suppress FOXO-Induced Apoptosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peripheral T Cells Ets-1 Maintains IL-7 Receptor Expression In
The Journal of Immunology Ets-1 Maintains IL-7 Receptor Expression in Peripheral T Cells Roland Grenningloh,*,† Tzong-Shyuan Tai,* Nicole Frahm,†,‡,1 Tomoyuki C. Hongo,‡ Adam T. Chicoine,‡ Christian Brander,†,‡,x,{ Daniel E. Kaufmann,†,‡,‖ and I-Cheng Ho*,† The expression of CD127, the IL-7–binding subunit of the IL-7 R, is tightly regulated during the development and activation of T cells and is reduced during chronic viral infection. However, the molecular mechanism regulating the dynamic expression of CD127 is still poorly understood. In this study, we report that the transcription factor Ets-1 is required for maintaining the expression of CD127 in murine peripheral T cells. Ets-1 binds to and activates the CD127 promoter, and its absence leads to reduced CD127 expression, attenuated IL-7 signaling, and impaired IL-7–dependent homeostatic proliferation of T cells. The expression of CD127 and Ets-1 is strongly correlated in human T cells. Both CD127 and Ets-1 expression are decreased in CD8+ T cells during HIV infection. In addition, HIV-associated loss of CD127 is only observed in Ets-1low effector memory and central memory but not in Ets-1high naive CD8+ T cells. Taken together, our data identify Ets-1 as a critical regulator of CD127 expression in T cells. The Journal of Immunology, 2011, 186: 969–976. nterleukin-7 signals are required for T cell development, GABPa or another Ets protein is responsible for maintaining maintaining the naive T cell pool, mounting proper primary CD127 expression in peripheral T cells is unknown. I responses, and inducing and maintaining CD4+ and CD8+ Ets-1 (E26 transformation-specific sequence) is the founding T cell memory (1–3). -

Single-Cell RNA-Sequencing-Based Crispri Screening Resolves Molecular Drivers of Early Human Endoderm Development
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2019-04-16 Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Ryan M. Genga University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Cell Biology Commons, Cells Commons, Developmental Biology Commons, Embryonic Structures Commons, Genetic Phenomena Commons, and the Nucleic Acids, Nucleotides, and Nucleosides Commons Repository Citation Genga RM, Kernfeld EM, Parsi KM, Parsons TJ, Ziller MJ, Maehr R. (2019). Single-Cell RNA-Sequencing- Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development. Open Access Articles. https://doi.org/10.1016/j.celrep.2019.03.076. Retrieved from https://escholarship.umassmed.edu/oapubs/3818 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Report Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Graphical Abstract Authors Ryan M.J. Genga, Eric M. Kernfeld, Krishna M. Parsi, Teagan J. Parsons, Michael J. Ziller, Rene´ Maehr Correspondence [email protected] In Brief Genga et al. utilize a single-cell RNA- sequencing-based CRISPR interference approach to screen transcription factors predicted to have a role in human definitive endoderm differentiation. -

Single Cell Transcriptomics Reveal Temporal Dynamics of Critical Regulators of Germ Cell Fate During Mouse Sex Determination
bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Single cell transcriptomics reveal temporal dynamics of critical regulators of germ 2 cell fate during mouse sex determination 3 Authors: Chloé Mayère1,2, Yasmine Neirijnck1,3, Pauline Sararols1, Chris M Rands1, 4 Isabelle Stévant1,2, Françoise Kühne1, Anne-Amandine Chassot3, Marie-Christine 5 Chaboissier3, Emmanouil T. Dermitzakis1,2, Serge Nef1,2,*. 6 Affiliations: 7 1Department of Genetic Medicine and Development, University of Geneva, 1211 Geneva, 8 Switzerland; 9 2iGE3, Institute of Genetics and Genomics of Geneva, University of Geneva, 1211 10 Geneva, Switzerland; 11 3Université Côte d'Azur, CNRS, Inserm, iBV, France; 12 Lead Contact: 13 *Corresponding Author: Serge Nef, 1 rue Michel-Servet CH-1211 Genève 4, 14 [email protected]. + 41 (0)22 379 51 93 15 Running Title: Single cell transcriptomics of germ cells 1 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 16 Abbreviations; 17 AGC: Adrenal Germ Cell 18 GC: Germ cell 19 OGC: Ovarian Germ Cell 20 TGC: Testicular Germ Cell 21 scRNA-seq: Single-cell RNA-Sequencing 22 DEG: Differentially Expressed Gene 23 24 25 Keywords: 26 Single-cell RNA-Sequencing (scRNA-seq), sex determination, ovary, testis, gonocytes, 27 oocytes, prospermatogonia, meiosis, gene regulatory network, germ cells, development, 28 RNA splicing 29 2 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Examination of the Transcription Factors Acting in Bone Marrow
THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY (PHD) Examination of the transcription factors acting in bone marrow derived macrophages by Gergely Nagy Supervisor: Dr. Endre Barta UNIVERSITY OF DEBRECEN DOCTORAL SCHOOL OF MOLECULAR CELL AND IMMUNE BIOLOGY DEBRECEN, 2016 Table of contents Table of contents ........................................................................................................................ 2 1. Introduction ............................................................................................................................ 5 1.1. Transcriptional regulation ................................................................................................... 5 1.1.1. Transcriptional initiation .................................................................................................. 5 1.1.2. Co-regulators and histone modifications .......................................................................... 8 1.2. Promoter and enhancer sequences guiding transcription factors ...................................... 11 1.2.1. General transcription factors .......................................................................................... 11 1.2.2. The ETS superfamily ..................................................................................................... 17 1.2.3. The AP-1 and CREB proteins ........................................................................................ 20 1.2.4. Other promoter specific transcription factor families ................................................... -

Apoptosis Induced by Proteasome Inhibition in Cancer Cells: Predominant Role of the P53/PUMA Pathway
Oncogene (2007) 26, 1681–1692 & 2007 Nature Publishing Group All rights reserved 0950-9232/07 $30.00 www.nature.com/onc ORIGINAL ARTICLE Apoptosis induced by proteasome inhibition in cancer cells: predominant role of the p53/PUMA pathway CG Concannon1, BF Koehler1,2, Claus Reimertz2, BM Murphy1, C Bonner1, N Thurow2, MW Ward1, AVillunger 3, AStrasser 4,DKo¨ gel2,5 and JHM Prehn1,5 1Department of Physiology and Medical Physics, Royal College of Surgeons in Ireland, Dublin, Ireland; 2Experimental Neurosurgery, Centre for Neurology and Neurosurgery, Johann Wolfgang Goethe University Clinics, Theodor-Stern-Kai 7, Frankfurt/Main, Germany; 3Division of Experimental Pathophysiology and Immunology, Biocenter, Innsbruck Medical University, Innsbruck, Austria and 4The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia The proteasome has emerged as a novel target for Introduction antineoplastic treatment of hematological malignancies and solid tumors, including those of the central nervous The correct functioning of the ubiquitin-proteasome system. To identify cell death pathways activated in pathway is essential for the degradation of the majority response to inhibition of the proteasome system in cancer of intracellular proteins. Several key regulatory proteins cells, we treated human SH-SY5Y neuroblastoma cells involved in cell proliferation and differentiation are with the selective proteasome inhibitor (PI) epoxomicin regulated by proteasome-mediated proteolysis resulting (Epoxo). Prolonged exposure to Epoxo was associated in the activation or inhibition of specific cell signaling with increased levels of poly-ubiquitinylated proteins and pathways (Adams, 2004a). The proteasome is also p53, release of cytochrome c from the mitochondria, and central to the regulation of cell death and apoptosis. -

Watsonjn2018.Pdf (1.780Mb)
UNIVERSITY OF CENTRAL OKLAHOMA Edmond, Oklahoma Department of Biology Investigating Differential Gene Expression in vivo of Cardiac Birth Defects in an Avian Model of Maternal Phenylketonuria A THESIS SUBMITTED TO THE GRADUATE FACULTY In partial fulfillment of the requirements For the degree of MASTER OF SCIENCE IN BIOLOGY By Jamie N. Watson Edmond, OK June 5, 2018 J. Watson/Dr. Nikki Seagraves ii J. Watson/Dr. Nikki Seagraves Acknowledgements It is difficult to articulate the amount of gratitude I have for the support and encouragement I have received throughout my master’s thesis. Many people have added value and support to my life during this time. I am thankful for the education, experience, and friendships I have gained at the University of Central Oklahoma. First, I would like to thank Dr. Nikki Seagraves for her mentorship and friendship. I lucked out when I met her. I have enjoyed working on this project and I am very thankful for her support. I would like thank Thomas Crane for his support and patience throughout my master’s degree. I would like to thank Dr. Shannon Conley for her continued mentorship and support. I would like to thank Liz Bullen and Dr. Eric Howard for their training and help on this project. I would like to thank Kristy Meyer for her friendship and help throughout graduate school. I would like to thank my committee members Dr. Robert Brennan and Dr. Lilian Chooback for their advisement on this project. Also, I would like to thank the biology faculty and staff. I would like to thank the Seagraves lab members: Jailene Canales, Kayley Pate, Mckayla Muse, Grace Thetford, Kody Harvey, Jordan Guffey, and Kayle Patatanian for their hard work and support. -

Figure S1. DMD Module Network. the Network Is Formed by 260 Genes from Disgenet and 1101 Interactions from STRING. Red Nodes Are the Five Seed Candidate Genes
Figure S1. DMD module network. The network is formed by 260 genes from DisGeNET and 1101 interactions from STRING. Red nodes are the five seed candidate genes. Figure S2. DMD module network is more connected than a random module of the same size. It is shown the distribution of the largest connected component of 10.000 random modules of the same size of the DMD module network. The green line (x=260) represents the DMD largest connected component, obtaining a z-score=8.9. Figure S3. Shared genes between BMD and DMD signature. A) A meta-analysis of three microarray datasets (GSE3307, GSE13608 and GSE109178) was performed for the identification of differentially expressed genes (DEGs) in BMD muscle biopsies as compared to normal muscle biopsies. Briefly, the GSE13608 dataset included 6 samples of skeletal muscle biopsy from healthy people and 5 samples from BMD patients. Biopsies were taken from either biceps brachii, triceps brachii or deltoid. The GSE3307 dataset included 17 samples of skeletal muscle biopsy from healthy people and 10 samples from BMD patients. The GSE109178 dataset included 14 samples of controls and 11 samples from BMD patients. For both GSE3307 and GSE10917 datasets, biopsies were taken at the time of diagnosis and from the vastus lateralis. For the meta-analysis of GSE13608, GSE3307 and GSE109178, a random effects model of effect size measure was used to integrate gene expression patterns from the two datasets. Genes with an adjusted p value (FDR) < 0.05 and an │effect size│>2 were identified as DEGs and selected for further analysis. A significant number of DEGs (p<0.001) were in common with the DMD signature genes (blue nodes), as determined by a hypergeometric test assessing the significance of the overlap between the BMD DEGs and the number of DMD signature genes B) MCODE analysis of the overlapping genes between BMD DEGs and DMD signature genes. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
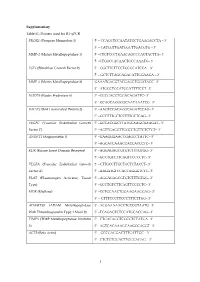
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Transcriptional Control of Tissue-Resident Memory T Cell Generation
Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Filip Cvetkovski All rights reserved ABSTRACT Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Tissue-resident memory T cells (TRM) are a non-circulating subset of memory that are maintained at sites of pathogen entry and mediate optimal protection against reinfection. Lung TRM can be generated in response to respiratory infection or vaccination, however, the molecular pathways involved in CD4+TRM establishment have not been defined. Here, we performed transcriptional profiling of influenza-specific lung CD4+TRM following influenza infection to identify pathways implicated in CD4+TRM generation and homeostasis. Lung CD4+TRM displayed a unique transcriptional profile distinct from spleen memory, including up-regulation of a gene network induced by the transcription factor IRF4, a known regulator of effector T cell differentiation. In addition, the gene expression profile of lung CD4+TRM was enriched in gene sets previously described in tissue-resident regulatory T cells. Up-regulation of immunomodulatory molecules such as CTLA-4, PD-1, and ICOS, suggested a potential regulatory role for CD4+TRM in tissues. Using loss-of-function genetic experiments in mice, we demonstrate that IRF4 is required for the generation of lung-localized pathogen-specific effector CD4+T cells during acute influenza infection. Influenza-specific IRF4−/− T cells failed to fully express CD44, and maintained high levels of CD62L compared to wild type, suggesting a defect in complete differentiation into lung-tropic effector T cells. -

Agonists and Knockdown of Estrogen Receptor Β Differentially Affect
Schüler-Toprak et al. BMC Cancer (2016) 16:951 DOI 10.1186/s12885-016-2973-y RESEARCH ARTICLE Open Access Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro Susanne Schüler-Toprak1*, Julia Häring1, Elisabeth C. Inwald1, Christoph Moehle2, Olaf Ortmann1 and Oliver Treeck1 Abstract Background: Estrogen receptor β (ERβ) is expressed in the majority of invasive breast cancer cases, irrespective of their subtype, including triple-negative breast cancer (TNBC). Thus, ERβ might be a potential target for therapy of this challenging cancer type. In this in vitro study, we examined the role of ERβ in invasion of two triple-negative breast cancer cell lines. Methods: MDA-MB-231 and HS578T breast cancer cells were treated with the specific ERβ agonists ERB-041, WAY200070, Liquiritigenin and 3β-Adiol. Knockdown of ERβ expression was performed by means of siRNA transfection. Effects on cellular invasion were assessed in vitro by means of a modified Boyden chamber assay. Transcriptome analyses were performed using Affymetrix Human Gene 1.0 ST microarrays. Pathway and gene network analyses were performed by means of Genomatix and Ingenuity Pathway Analysis software. Results: Invasiveness of MBA-MB-231 and HS578T breast cancer cells decreased after treatment with ERβ agonists ERB-041 and WAY200070. Agonists Liquiritigenin and 3β-Adiol only reduced invasion of MDA-MB-231 cells. Knockdown of ERβ expression increased invasiveness of MDA-MB-231 cells about 3-fold. Transcriptome and pathway analyses revealed that ERβ knockdown led to activation of TGFβ signalling and induced expression of a network of genes with functions in extracellular matrix, tumor cell invasion and vitamin D3 metabolism.