Subunit Gene Expression Through Smad-Binding and Homeobox Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single-Cell RNA-Sequencing-Based Crispri Screening Resolves Molecular Drivers of Early Human Endoderm Development
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2019-04-16 Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Ryan M. Genga University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Cell Biology Commons, Cells Commons, Developmental Biology Commons, Embryonic Structures Commons, Genetic Phenomena Commons, and the Nucleic Acids, Nucleotides, and Nucleosides Commons Repository Citation Genga RM, Kernfeld EM, Parsi KM, Parsons TJ, Ziller MJ, Maehr R. (2019). Single-Cell RNA-Sequencing- Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development. Open Access Articles. https://doi.org/10.1016/j.celrep.2019.03.076. Retrieved from https://escholarship.umassmed.edu/oapubs/3818 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Report Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Graphical Abstract Authors Ryan M.J. Genga, Eric M. Kernfeld, Krishna M. Parsi, Teagan J. Parsons, Michael J. Ziller, Rene´ Maehr Correspondence [email protected] In Brief Genga et al. utilize a single-cell RNA- sequencing-based CRISPR interference approach to screen transcription factors predicted to have a role in human definitive endoderm differentiation. -

Single Cell Transcriptomics Reveal Temporal Dynamics of Critical Regulators of Germ Cell Fate During Mouse Sex Determination
bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Single cell transcriptomics reveal temporal dynamics of critical regulators of germ 2 cell fate during mouse sex determination 3 Authors: Chloé Mayère1,2, Yasmine Neirijnck1,3, Pauline Sararols1, Chris M Rands1, 4 Isabelle Stévant1,2, Françoise Kühne1, Anne-Amandine Chassot3, Marie-Christine 5 Chaboissier3, Emmanouil T. Dermitzakis1,2, Serge Nef1,2,*. 6 Affiliations: 7 1Department of Genetic Medicine and Development, University of Geneva, 1211 Geneva, 8 Switzerland; 9 2iGE3, Institute of Genetics and Genomics of Geneva, University of Geneva, 1211 10 Geneva, Switzerland; 11 3Université Côte d'Azur, CNRS, Inserm, iBV, France; 12 Lead Contact: 13 *Corresponding Author: Serge Nef, 1 rue Michel-Servet CH-1211 Genève 4, 14 [email protected]. + 41 (0)22 379 51 93 15 Running Title: Single cell transcriptomics of germ cells 1 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 16 Abbreviations; 17 AGC: Adrenal Germ Cell 18 GC: Germ cell 19 OGC: Ovarian Germ Cell 20 TGC: Testicular Germ Cell 21 scRNA-seq: Single-cell RNA-Sequencing 22 DEG: Differentially Expressed Gene 23 24 25 Keywords: 26 Single-cell RNA-Sequencing (scRNA-seq), sex determination, ovary, testis, gonocytes, 27 oocytes, prospermatogonia, meiosis, gene regulatory network, germ cells, development, 28 RNA splicing 29 2 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. -

Watsonjn2018.Pdf (1.780Mb)
UNIVERSITY OF CENTRAL OKLAHOMA Edmond, Oklahoma Department of Biology Investigating Differential Gene Expression in vivo of Cardiac Birth Defects in an Avian Model of Maternal Phenylketonuria A THESIS SUBMITTED TO THE GRADUATE FACULTY In partial fulfillment of the requirements For the degree of MASTER OF SCIENCE IN BIOLOGY By Jamie N. Watson Edmond, OK June 5, 2018 J. Watson/Dr. Nikki Seagraves ii J. Watson/Dr. Nikki Seagraves Acknowledgements It is difficult to articulate the amount of gratitude I have for the support and encouragement I have received throughout my master’s thesis. Many people have added value and support to my life during this time. I am thankful for the education, experience, and friendships I have gained at the University of Central Oklahoma. First, I would like to thank Dr. Nikki Seagraves for her mentorship and friendship. I lucked out when I met her. I have enjoyed working on this project and I am very thankful for her support. I would like thank Thomas Crane for his support and patience throughout my master’s degree. I would like to thank Dr. Shannon Conley for her continued mentorship and support. I would like to thank Liz Bullen and Dr. Eric Howard for their training and help on this project. I would like to thank Kristy Meyer for her friendship and help throughout graduate school. I would like to thank my committee members Dr. Robert Brennan and Dr. Lilian Chooback for their advisement on this project. Also, I would like to thank the biology faculty and staff. I would like to thank the Seagraves lab members: Jailene Canales, Kayley Pate, Mckayla Muse, Grace Thetford, Kody Harvey, Jordan Guffey, and Kayle Patatanian for their hard work and support. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
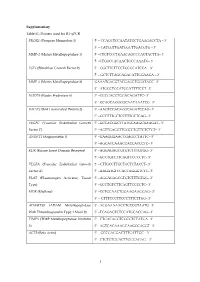
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -

Single Cell Profiling of CRISPR/Cas9-Induced OTX2 Deficient Retinas Reveals Fate Switch from Restricted Progenitors
bioRxiv preprint doi: https://doi.org/10.1101/538710; this version posted February 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Single cell profiling of CRISPR/Cas9-induced OTX2 deficient retinas reveals fate switch from restricted progenitors Miruna G. Ghinia Tegla1, Diego F. Buenaventura1, 2, Diana Y. Kim1, Cassandra Thakurdin1, Kevin C. Gonzalez1, 3, Mark M. Emerson1,2* 1 Department of Biology, The City College of New York, City University of New York, New York, NY, 10031; United States of America 2 Biology Ph.D. Program, Graduate Center, City University of New York, New York, NY, 10031; United States of America 3 Present address: Doctoral Program in Neurobiology and Behavior, Columbia University, New York, NY 10032; United States of America *Corresponding author: [email protected] Email addresses: Miruna G. Ghinia Tegla: [email protected] Diego F. Buenaventura: [email protected] Diana Y. Kim: [email protected] Cassandra Thakurdin: [email protected] Kevin C. Gonzalez: [email protected] Mark M. Emerson: [email protected] Running title: Single cell analysis of retinal cell fate changes induced by OTX2 mutagenesis bioRxiv preprint doi: https://doi.org/10.1101/538710; this version posted February 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Development of the vertebrate eye, like many developmental systems, depends on genes that are used iteratively in multiple distinct processes. -

The Id-Protein Family in Developmental and Cancer-Associated Pathways Cornelia Roschger and Chiara Cabrele*
Roschger and Cabrele Cell Communication and Signaling (2017) 15:7 DOI 10.1186/s12964-016-0161-y REVIEW Open Access The Id-protein family in developmental and cancer-associated pathways Cornelia Roschger and Chiara Cabrele* Abstract Inhibitors of DNA binding and cell differentiation (Id) proteins are members of the large family of the helix-loop- helix (HLH) transcription factors, but they lack any DNA-binding motif. During development, the Id proteins play a key role in the regulation of cell-cycle progression and cell differentiation by modulating different cell-cycle regulators both by direct and indirect mechanisms. Several Id-protein interacting partners have been identified thus far, which belong to structurally and functionally unrelated families, including, among others, the class I and II bHLH transcription factors, the retinoblastoma protein and related pocket proteins, the paired-box transcription factors, and the S5a subunit of the 26 S proteasome. Although the HLH domain of the Id proteins is involved in most of their protein-protein interaction events, additional motifs located in their N-terminal and C-terminal regions are required for the recognition of diverse protein partners. The ability of the Id proteins to interact with structurally different proteins is likely to arise from their conformational flexibility: indeed, these proteins contain intrinsically disordered regions that, in the case of the HLH region, undergo folding upon self- or heteroassociation. Besides their crucial role for cell-fate determination and cell-cycle progression during development, other important cellular events have been related to the Id-protein expression in a number of pathologies. Dysregulated Id-protein expression has been associated with tumor growth, vascularization, invasiveness, metastasis, chemoresistance and stemness, as well as with various developmental defects and diseases. -

At the X-Roads of Sex and Genetics in Pulmonary Arterial Hypertension
G C A T T A C G G C A T genes Review At the X-Roads of Sex and Genetics in Pulmonary Arterial Hypertension Meghan M. Cirulis 1,2,* , Mark W. Dodson 1,2, Lynn M. Brown 1,2, Samuel M. Brown 1,2, Tim Lahm 3,4,5 and Greg Elliott 1,2 1 Division of Pulmonary, Critical Care and Occupational Medicine, University of Utah, Salt Lake City, UT 84132, USA; [email protected] (M.W.D.); [email protected] (L.M.B.); [email protected] (S.M.B.); [email protected] (G.E.) 2 Division of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Salt Lake City, UT 84107, USA 3 Division of Pulmonary, Critical Care, Sleep and Occupational Medicine, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA; [email protected] 4 Richard L. Roudebush Veterans Affairs Medical Center, Indianapolis, IN 46202, USA 5 Department of Anatomy, Cell Biology & Physiology, Indiana University School of Medicine, Indianapolis, IN 46202, USA * Correspondence: [email protected]; Tel.: +1-801-581-7806 Received: 29 September 2020; Accepted: 17 November 2020; Published: 20 November 2020 Abstract: Group 1 pulmonary hypertension (pulmonary arterial hypertension; PAH) is a rare disease characterized by remodeling of the small pulmonary arteries leading to progressive elevation of pulmonary vascular resistance, ultimately leading to right ventricular failure and death. Deleterious mutations in the serine-threonine receptor bone morphogenetic protein receptor 2 (BMPR2; a central mediator of bone morphogenetic protein (BMP) signaling) and female sex are known risk factors for the development of PAH in humans. -

FOXP1 Acts Through a Negative Feedback Loop to Suppress FOXO-Induced Apoptosis
Cell Death and Differentiation (2013) 20, 1219–1229 & 2013 Macmillan Publishers Limited All rights reserved 1350-9047/13 www.nature.com/cdd FOXP1 acts through a negative feedback loop to suppress FOXO-induced apoptosis R van Boxtel1,5, C Gomez-Puerto1,6, M Mokry2,6, A Eijkelenboom3, KE van der Vos1, EES Nieuwenhuis2, BMT Burgering3, EW-F Lam4 and PJ Coffer*,1,2 Transcriptional activity of Forkhead box transcription factor class O (FOXO) proteins can result in a variety of cellular outcomes depending on cell type and activating stimulus. These transcription factors are negatively regulated by the phosphoinositol 3-kinase (PI3K)–protein kinase B (PKB) signaling pathway, which is thought to have a pivotal role in regulating survival of tumor cells in a variety of cancers. Recently, it has become clear that FOXO proteins can promote resistance to anti-cancer therapeutics, designed to inhibit PI3K–PKB activity, by inducing the expression of proteins that provide feedback at different levels of this pathway. We questioned whether such a feedback mechanism may also exist directly at the level of FOXO-induced transcription. To identify critical modulators of FOXO transcriptional output, we performed gene expression analyses after conditional activation of key components of the PI3K–PKB–FOXO signaling pathway and identified FOXP1 as a direct FOXO transcriptional target. Using chromatin immunoprecipitation followed by next-generation sequencing, we show that FOXP1 binds enhancers that are pre-occupied by FOXO3. By sequencing the transcriptomes of cells in which FOXO is specifically activated in the absence of FOXP1, we demonstrate that FOXP1 can modulate the expression of a specific subset of FOXO target genes, including inhibiting expression of the pro-apoptotic gene BIK. -

The Role of Inhibitors of Differentiation Proteins ID1 and ID3 in Breast Cancer Metastasis
The role of Inhibitors of Differentiation proteins ID1 and ID3 in breast cancer metastasis Wee Siang Teo A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy St Vincent’s Clinical School, Faculty of Medicine The University of New South Wales Cancer Research Program The Garvan Institute of Medical Research Sydney, Australia March, 2014 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Teo First name: Wee Siang Abbreviation for degree as given in the University calendar: PhD (Medicine) School: St Vincent’s Clinical School Faculty: Faculty of Medicine Title: The role of Inhibitors of Differentiation proteins ID1 and ID3 in breast cancer metastasis Abstract 350 words maximum: (PLEASE TYPE) Breast cancer is a leading cause of cancer death in women. While locally-confined breast cancer is generally curable, the survival of patients with metastatic breast cancer is very poor. Treatment for metastatic breast cancer is palliative not curative due to the lack of targeted therapies. Metastasis is a complex process that still remains poorly understood, thus a detailed understanding of the biological complexity that underlies breast cancer metastasis is essential in reducing the lethality of this disease. The Inhibitor of Differentiation proteins 1 and 3 (ID1/3) are transcriptional regulators that control many cell fate and developmental processes and are often deregulated in cancer. ID1/3 are required and sufficient for the metastasis of breast cancer in experimental models. However, the mechanisms by which ID1/3 mediate metastasis in breast cancer remain to be determined. Little is known about pathways regulated by ID1/3 in breast cancer as well as their functional role in the multiple steps of metastatic progression. -

ID1 Mediates Escape from TGF-Β Tumor Suppression in Pancreatic Cancer
Author Manuscript Published OnlineFirst on October 3, 2019; DOI: 10.1158/2159-8290.CD-19-0529 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. ID1 mediates escape from TGF-β tumor suppression in pancreatic cancer Yun-Han Huang1,2,3, Jing Hu1, Fei Chen1, Nicolas Lecomte4, Harihar Basnet1, Charles J. David1,10, Matthew D. Witkin5, Peter J. Allen6, Steven D. Leach4,6,7,9, Travis J. Hollmann7,8, Christine A. Iacobuzio-Donahue4,7,8, and Joan Massagué1* 1Cancer Biology and Genetics Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065 2Weill Cornell/Sloan Kettering/Rockefeller Tri-Institutional MD-PhD Program, New York, NY 10065 3Gerstner Sloan Kettering Graduate School of Biomedical Sciences, New York, NY 10065 4The David M. Rubinstein Center for Pancreatic Cancer Research, Memorial Sloan Kettering Cancer Center, New York, NY 10065 5Center for Epigenetics Research, Memorial Sloan Kettering Cancer Center, New York, NY 10065 6Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10065 7Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY 10065 8Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065 9Present address: Department of Molecular and Systems Biology, Dartmouth Geisel School of Medicine, 1 Rope Ferry Road, Hanover, NH 03755-1404 10Present address: Tsinghua University School of Medicine, Department of Basic Sciences, Medical Sciences Building D106, Haidian District, Beijing, China, 100084 Running title: ID1 mediates escape from TGF-β tumor suppression in PDA Keywords: TGF-β, pancreatic cancer, ID1, tumor suppression, EMT Financial support: National Cancer Institute grants R01-CA34610 (JM) and P30-CA008748 (MSKCC), and Predoctoral Fellowship F30-CA203238 (YH).