The University of Oklahoma Graduate College
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Tetraethyllead Poisoning
Arch. Toxikol. 24, 283--291 (1969) Acute Tetraethyllead Poisoning M. STASIK, Z. BYCZKOWSKA, S. SZENDZIKOWSKI,and Z. FIEDOttCZUK Clinical and Pathomorphological Department, Institute of Occupational Medicine, L6@, Poland, Department of Forensic Medicine, Medical Academy, L6dA l%eceived September 16, 1968 Summary. Four cases of accidental poisoning with tetraethyllead are described. Three out of four of the patients died. In the first case, pure ethyl fluid was accidentally ingested. Dominating the clinical picture of this patient were signs of greatly elevated intracranial pressure. Three other persons were poisoned as a group. They unknowingly inhaled tetra- ethyllead contained in a paint solvent they used. In these three cases, the intoxication manifested itself predominantly as a mental disorder suggestive of schizophrenia. Gross and microscopic changes observed in the fatal cases gave evidence of a capillary vascular lesion, particularly involving the vessels of the CNS. Liver damage and less severe damage to the heart muscle and kidney parenchyma were also noted. The distribution as well as the extent of the above mentioned lesions correlate approximately with the distribution and concentration of triethyllead in the various internal organs. Key- Words: Tetraethyllead -- Mental Disorder -- Damage to Parenchymatous Organs. Zusammen/assung. Die Verfasser berichten fiber 4 F/ille, yon denen 3 tSdlich waren, zuf~lliger Vergiftungen durch das sog. Ethylfluid, das Bleitetra~thyl enthElt. Im ersten Fall trat die t6dliche Vergiftung infolge irrtfimlich getrunkenem Ethylfluid auf. Als klinisches Symptom entstand erh6hter intrakranieller Druck. In den drei n~ehsten F~llen besa6 die Vergiftung einen kollektiven Charakter und war durch den Respirationstrakt zustande gekommen; zwei von den Vergifteten sind gestorben. -

Technical Note TN-106 a Guideline for PID Instriment Response
Technical Note TN-106 05/15/VK A GUIDELINE FOR PID INSTRUMENT RESPONSE CORRECTION FACTORS AND IONIZATION ENERGIES* Example 1: RAE Systems PIDs can be used for the detection of a wide variety of With the unit calibrated to read isobutylene equivalents, the reading gases that exhibit different responses. In general, any compound with is 10 ppm with a 10.6 eV lamp. The gas being measured is butyl ionization energy (IE) lower than that of the lamp photons can acetate, which has a correction factor of 2.6. Multiplying 10 by 2.6 be measured.* The best way to calibrate a PID to different compounds gives an adjusted butyl acetate value of 26 ppm. Similarly, if the is to use a standard of the gas of interest. However, correction factors gas being measured were trichloroethylene (CF = 0.54), the adjusted have been determined that enable the user to quantify a large number value with a 10 ppm reading would be 5.4 ppm. of chemicals using only a single calibration gas, typically isobutylene. In our PIDs, correction factors can be used in one of three ways: Example 2: With the unit calibrated to read isobutylene equivalents, the reading 1. Calibrate the monitor with isobutylene in the usual fashion to is 100 ppm with a 10.6 eV lamp. The gas measured is m-xylene read in isobutylene equivalents. Manually multiply the reading (CF = 0.43). After downloading this factor, the unit should read about by the correction factor (CF) to obtain the concentration of 43 ppm when exposed to the same gas, and thus read directly in the gas being measured. -

United States Patent Office Patented Aug
2,849,302 United States Patent Office Patented Aug. 26, 1953 thasaraywarrasants, 2. east one hydrogen atom attached thereto. That is, each of the tertiary halogens is capable of forming hydro 2,849,392 gen halide with a hydrogen atom attached to a different ANTIKNoCK COMPOSITIONS carbon atom which is in a position alpha to the halogen 5 bearing carbon. In this description the term tertiary car Raymond G. Lyben, Detroit, Mich., assigner to Ethy bon atom is defined as a carbon atom which has three Corporation, New York, N. Y., a corporation of Dear other carbon atoms attached thereto by single bonds. Ware Thus, the novel scavenging agents of this invention are No Drawing. Application May 31, 955 certain chlorohydrocarbons, bromohydrocarbons and Seria Rio. 52,284 chlorobromohydrocarbons. The halohydrocarbon scav enging agents of this invention can be derived from al 7 Clains. (C. 44-69) kanes, cycloalkanes, alkenes, cycloalkenes, and hydrocar bon-substituted derivatives thereof. The smallest hydro carbon radical which can provide a scavenger of this in This invention relates to improved antiknock composi s vention contains five carbon atoms; e. g., 1,2-dihalo-1,2- tions. These compositicins encompass antiknock fluids dimethylcyclopropane. In order to provide scavengers and leaded fuels. In particular, this invention relates to having the desirable volatility characteristics with respect a class of hydrocarbons having a particular molecular to the lead antiknock agent, I prefer to employ uniform structure for use as a scavenger with lead antiknock con ly stable scavengers having up to 20 carbon atoms. pounds. Thus, it will be seen that the carbon content of my scav With the discovery of the antiknock effectiveness of or engers ranges from 5 to 20 carbons per molecule. -
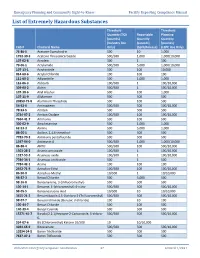
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Cumulative Cross Index to Iarc Monographs
RADIATION volume 100 D A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 2-9 June 2009 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns CUMULATIVE CROSS INDEX TO IARC MONOGRAPHS The volume, page and year of publication are given. References to corrigenda are given in parentheses. A A-α-C .............................................................40, 245 (1986); Suppl. 7, 56 (1987) Acenaphthene ........................................................................92, 35 (2010) Acepyrene ............................................................................92, 35 (2010) Acetaldehyde ........................36, 101 (1985) (corr. 42, 263); Suppl. 7, 77 (1987); 71, 319 (1999) Acetaldehyde associated with the consumption of alcoholic beverages ..............100E, 377 (2012) Acetaldehyde formylmethylhydrazone (see Gyromitrin) Acetamide .................................... 7, 197 (1974); Suppl. 7, 56, 389 (1987); 71, 1211 (1999) Acetaminophen (see Paracetamol) Aciclovir ..............................................................................76, 47 (2000) Acid mists (see Sulfuric acid and other strong inorganic acids, occupational exposures to mists and vapours from) Acridine orange ...................................................16, 145 (1978); Suppl. 7, 56 (1987) Acriflavinium chloride ..............................................13, 31 (1977); Suppl. 7, -

Tetraethyllead Is a Deadly Toxic Chemical Substance Giving Rise to Severe Psychotic Manifestations. for Its Excellent Properties
Industrial Health, 1986, 24, 139-150. Determination of Triethyllead, Diethyllead and Inorganic Lead in Urine by Atomic Absorption Spectrometry Fumio ARAI Department of Public Health St. Marianna University School of Medicine 2095 Sugao, Miyamae-ku, Kawasaki 213, Japan (Received March 10, 1986 and in revised form May 21, 1986) Abstract : A method was developed for the sequential extraction of tetraethyllead (Et4Pb), triethyllead (Et3Pb+), diethyllead (Et2Pb2+) and inorganic lead (Pb2+) from one urine sample with methyl isobutyl ketone and the subsequent sequential determination of the respective species of lead by flame and flameless atomic ab- sorption spectrometry. When 40 ml of a urine sample to which 2 ƒÊg of Pb of each of Et4Pb, Et3Pb+, Et2Pb2+ or Pb2+ had been experimentally added was assayed for the respective species of lead by flame atomic absorption spectrometry, ten repetitions of the assay gave a mean recovery rate of 98% for each of Et4Pb, Et3Pb+, and Et2Pb2+, and 99% for Pb2+, with a coefficient of variation of 2.0% for Et4Pb, 0.7% for Et3Pb+ and Pb2+, 2.6% for Et2Pb2+, and a detection limit of 4 ƒÊg of Pb/L for Et4Pb, 3 ƒÊg of Pb/L for Et3Pb+, and 5 ƒÊg of Pb/L for each of Et2Pb2+ and Pb2+. Examination of urine samples from a patient with tetraethyllead poisoning 22 days after exposure to the lead revealed that the total lead output was made up of about 51% Pb2+, about 43% Et2Pb2+, and about 6% Et3Pb+ but no Et4Pb. Ad- ministration of calcium ethylenediaminetetraacetic acid (Ca-EDTA) was followed by no increased urinary excretion of Et3Pb+ or Et2Pb2+. -

Leaded Avgas Safety Data Sheet According to Federal Register / Vol
Leaded Avgas Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Revision Date : 01/22/2014 Version : 1.0 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY 1.1. Product Identifier Product Form: Mixture Product Name: Leaded Avgas Synonyms: Avgas; Avgas100LL; Aviation Gasoline; Avgas 100LL; DOT - Gasoline, 3, II Product Group: Commercial product 1.2. Intended Use of the Product Use of the Substance/Mixture: Aviation fuel. For professional use only. 1.3. Name, Address, and Telephone of the Responsible Party Customer EPIC Aviation, LLC P.O. Box 12249 Salem, OR 97309 T 866-501-3742 www.EPICaviationllc.com 1.4. Emergency Telephone Number Emergency Number : Within USA and Canada: 800-424-9300 Outside USA and Canada: +1 703-527-3887 (Collect Calls Accepted) For Chemical Emergency, Spill, Leak, Fire, Exposure, or Accident, call CHEMTREC – Day or Night SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture Classification (GHS-US) Flam. Liq. 1 H224 Skin Irrit. 2 H315 Eye Irrit. 2A H319 Muta. 1B H340 Carc. 1A H350 Repr. 1A H360 STOT SE 3 H336 STOT RE 1 H372 Asp. Tox. 1 H304 Aquatic Acute 2 H401 Aquatic Chronic 2 H411 2.2. Label Elements GHS-US Labeling Hazard Pictograms (GHS-US) : GHS02 GHS07 GHS08 GHS09 Signal Word (GHS-US) : Danger Hazard Statements (GHS-US) : H224 - Extremely flammable liquid and vapor H304 - May be fatal if swallowed and enters airways H315 - Causes skin irritation H319 - Causes serious eye irritation H336 - May cause drowsiness or dizziness H340 - May cause genetic defects H350 - May cause cancer H360 - May damage fertility or the unborn child 01/22/2014 EN (English US) 1/21 Leaded Avgas Safety Data Sheet according to Federal Register / Vol. -

Cumulative Cross Index to Iarc Monographs
PERSONAL HABITS AND INDOOR COMBUSTIONS volume 100 e A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 29 September-6 October 2009 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns CUMULATIVE CROSS INDEX TO IARC MONOGRAPHS The volume, page and year of publication are given. References to corrigenda are given in parentheses. A A-α-C .............................................................40, 245 (1986); Suppl. 7, 56 (1987) Acenaphthene ........................................................................92, 35 (2010) Acepyrene ............................................................................92, 35 (2010) Acetaldehyde ........................36, 101 (1985) (corr. 42, 263); Suppl. 7, 77 (1987); 71, 319 (1999) Acetaldehyde associated with the consumption of alcoholic beverages ..............100E, 377 (2012) Acetaldehyde formylmethylhydrazone (see Gyromitrin) Acetamide .................................... 7, 197 (1974); Suppl. 7, 56, 389 (1987); 71, 1211 (1999) Acetaminophen (see Paracetamol) Aciclovir ..............................................................................76, 47 (2000) Acid mists (see Sulfuric acid and other strong inorganic acids, occupational exposures to mists and vapours from) Acridine orange ...................................................16, 145 (1978); Suppl. 7, 56 (1987) Acriflavinium chloride ..............................................13, -

Degradation and Metabolism of Tetraethyllead in Soils
Journal of Industrial Microbiology (1995] 14, 312-318 1995 Society for Industrial Microbiology 0169-4146/95/S12,00 Degradation and metabolism of tetraethyllead in soils Li-Tse Ou, Wei Jing and John E. Thomas Soil and Water Science Department, University of Florida, Gainesville, FL 32611, USA (Received 31 March 1994; accepted 19 September 1994) Key words: Tetraethyllead; Antiknock agent; Biological degradation; Chemical degradation; Metabolites; Soils Downloaded from https://academic.oup.com/jimb/article/14/3-4/312/5988466 by guest on 30 September 2021 SUMMARY The objective of this study was to determine the disappearanceof the ieaded gasoline enhancer tetraethyllead (TEL), formation of degradation products, and mass balance in nonsterile and autoclaved Leon and Madison soils. EthyI-1J4C-labeledTEL was used so that mineralizationrates of TEL and mass balance could be determined. ~4C-TELin nonsterile and autoclaved surface and subsurface samples of the two soils disappeared rapidly, and ionic ethyllead products, water soluble nonlead organic products and bound residues were rapidly formed. A small fraction (<7.74%) of 14C-TELin nonsterile soil samples was mineralized to 14CO2 in 28 days. Triethyllead(TREL) was the major ionic ethylleadproduct detected in both nonsterileand autoclaved soils; diethyllead (DEL) was occasionally detected. Recovery of ~4C from mass balance studies for all nonsterile and autoclaved soil samples after 28 days of incubation was poor, less than 50% of the 14C applied. It appears that unknown volatile and/or gaseous organic products were the major degradation products of TEL in soils. Based on the observationsof more rapid initial disappearanceof ~4C-TEL,more rapid formationand more rapid disappearanceof 14C-DEL, and occurrenceof t*COzproduction in nonsterilesoils, it was concluded that both biological and chemical degradation contributedto the degradation of TEL in soils, with chemical degradation being the major factor. -

The Potential for Ground Water Contamination by the Gasoline Lead Scavengers Ethylene Dibromide and 1,2-Dichloroethane
The Potential for Ground Water Contamination by the Gasoline Lead Scavengers Ethylene Dibromide and 1,2-Dichloroethane by Ronald W. Falta Abstract Ethylene dibromide (EDB) is a synthetic organic chemical that was produced in large amounts for use as a leaded gasoline additive and pesticide. The chlorinated solvent 1,2-dichlorethane (1,2-DCA) is widely used in the chemical industry, and was also added to leaded gasoline. EDB and 1,2-DCA are classified as probable human carcinogens by the U.S. Environmental Pro- tection Agency (U.S. EPA), and EDB’s use as a pesticide was suspended in 1984. The current U.S. EPA maximum contami- nant level (MCL) for EDB in drinking water is 0.05 µg/L, and the MCL for 1,2-DCA is 5 µg/L. EDB has proven to be both mobile and persistent in ground water, and contamination of ground water by EDB was documented in several states beginning in the early 1980s. The majority of this contamination is attributed to agricultural uses of EDB; however, ~90% of the EDB pro- duced was used as a leaded gasoline additive and it was present in virtually all leaded gasoline sold in the United States. 1,2- DCA is commonly found as a ground water contaminant, and it is both mobile and persistent. Past investigations and remediation efforts at sites contaminated by leaded gasoline have rarely addressed the potential for EDB or 1,2-DCA contami- nation. For this reason, there is a substantial likelihood that undetected EDB and 1,2-DCA plumes above the MCL may exist at many sites where leaded gasoline leaked or spilled. -

The Worldwide Problem of Lead in Petrol Philip J
Editorials The worldwide problem of lead in petrol Philip J. Landrigan1 The use of lead as a petrol additive has mean blood lead level (6). Similar effects earlier this year, called specifically for the been a catastrophe for public health. were recorded in Western Europe, removal of lead from petrol. This call Leaded petrol has caused more exposure Australia, Canada, New Zealand and resonates with the theme of World Health to lead than any other source worldwide. South Africa (7). In a number of devel- Day 2003, which is healthy environments By contaminating air, dust, soil, drinking- oping countries too, including China, for children. water and food crops, it has caused El Salvador, India, Mexico and Thailand, Precaution is the lesson to be learnt harmfully high human blood lead levels declines in blood lead levels have followed from the history of lead in petrol. The around the world, especially in children (1). the removal of lead from petrol (8). worldwide dissemination of tetraethyl- Tetraethyllead was first added to Nearly 50 nations have now renounced lead is a classic example of our excessive petrol in 1922, to improve engine perfor- the use of lead in petrol, and more are willingness to adopt a promising but mance. Soon after production began in planning such action in the next five years. unproven new technology without heed Bayway, New Jersey, USA, an outbreak of Worldwide, unleaded petrol now to its possible consequences. We made acute neuropsychiatric disease appeared accounts for 80% of total sales (1). This is the same error with chlorofluorocarbon among workers, 80% of whom developed a triumph for public health. -

Tetraethyllead
Poison Facts: Low Chemicals: Tetraethyllead Properties of the Chemical Tetraethyllead is an oily, colorless liquid with a characteristic musty, fruity, even pleasant odor. It releases a poisonous, flammable vapor. The product burns with an orange-colored flame with green margins. It does not react with water. Uses of the Chemical Tetraethyllead has been used since 1923 as a petroleum octane booster and “anti-knock” agent in both automotive and aviation fuels. In 1978, the United States placed a gradual ban on the chemical’s use in automotive fuels. By the end of 1995, the chemical was replaced in automotive fuels by methyl-tert-butyl ether. However, tetraethyllead is still allowed in aviation fuels. A typical aviation mix includes up to 62 percent tetraethyllead. Absorption, Distribution, Metabolism and Excretion (ADME) Tetraethyllead is well-absorbed by all routes of exposure. It is an organic lead that is distributed throughout the brain and lipid-rich organs. It is rapidly metabolized to triethyl lead, another organic lead. Triethyl lead is slowly (from weeks to months) metabolized into an inorganic lead. Inorganic leads tend to be deposited into the bones, where they remain stable. The inorganic leads are gradually excreted by the kidneys. Clinical Effects of Acute Exposure • Ocular exposures: Eye exposure produces pain, itching, conjunctivitis, blurred vision and burns. • Dermal exposures: Tetraethyllead is readily absorbed through intact skin. It produces itching, burning and transient redness. On occasion, skin may begin to look pale. • Inhalation exposures: Inhalation is the major route of exposure; however, symptoms of tetraethyllead exposure cannot be separated from the also-toxic solvent mixture it is associated with.