Read Book « Fuel Additives ~ GYNZNZIFNQ7W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Tetraethyllead Poisoning
Arch. Toxikol. 24, 283--291 (1969) Acute Tetraethyllead Poisoning M. STASIK, Z. BYCZKOWSKA, S. SZENDZIKOWSKI,and Z. FIEDOttCZUK Clinical and Pathomorphological Department, Institute of Occupational Medicine, L6@, Poland, Department of Forensic Medicine, Medical Academy, L6dA l%eceived September 16, 1968 Summary. Four cases of accidental poisoning with tetraethyllead are described. Three out of four of the patients died. In the first case, pure ethyl fluid was accidentally ingested. Dominating the clinical picture of this patient were signs of greatly elevated intracranial pressure. Three other persons were poisoned as a group. They unknowingly inhaled tetra- ethyllead contained in a paint solvent they used. In these three cases, the intoxication manifested itself predominantly as a mental disorder suggestive of schizophrenia. Gross and microscopic changes observed in the fatal cases gave evidence of a capillary vascular lesion, particularly involving the vessels of the CNS. Liver damage and less severe damage to the heart muscle and kidney parenchyma were also noted. The distribution as well as the extent of the above mentioned lesions correlate approximately with the distribution and concentration of triethyllead in the various internal organs. Key- Words: Tetraethyllead -- Mental Disorder -- Damage to Parenchymatous Organs. Zusammen/assung. Die Verfasser berichten fiber 4 F/ille, yon denen 3 tSdlich waren, zuf~lliger Vergiftungen durch das sog. Ethylfluid, das Bleitetra~thyl enthElt. Im ersten Fall trat die t6dliche Vergiftung infolge irrtfimlich getrunkenem Ethylfluid auf. Als klinisches Symptom entstand erh6hter intrakranieller Druck. In den drei n~ehsten F~llen besa6 die Vergiftung einen kollektiven Charakter und war durch den Respirationstrakt zustande gekommen; zwei von den Vergifteten sind gestorben. -

Technical Note TN-106 a Guideline for PID Instriment Response
Technical Note TN-106 05/15/VK A GUIDELINE FOR PID INSTRUMENT RESPONSE CORRECTION FACTORS AND IONIZATION ENERGIES* Example 1: RAE Systems PIDs can be used for the detection of a wide variety of With the unit calibrated to read isobutylene equivalents, the reading gases that exhibit different responses. In general, any compound with is 10 ppm with a 10.6 eV lamp. The gas being measured is butyl ionization energy (IE) lower than that of the lamp photons can acetate, which has a correction factor of 2.6. Multiplying 10 by 2.6 be measured.* The best way to calibrate a PID to different compounds gives an adjusted butyl acetate value of 26 ppm. Similarly, if the is to use a standard of the gas of interest. However, correction factors gas being measured were trichloroethylene (CF = 0.54), the adjusted have been determined that enable the user to quantify a large number value with a 10 ppm reading would be 5.4 ppm. of chemicals using only a single calibration gas, typically isobutylene. In our PIDs, correction factors can be used in one of three ways: Example 2: With the unit calibrated to read isobutylene equivalents, the reading 1. Calibrate the monitor with isobutylene in the usual fashion to is 100 ppm with a 10.6 eV lamp. The gas measured is m-xylene read in isobutylene equivalents. Manually multiply the reading (CF = 0.43). After downloading this factor, the unit should read about by the correction factor (CF) to obtain the concentration of 43 ppm when exposed to the same gas, and thus read directly in the gas being measured. -

United States Patent Office Patented Aug
2,849,302 United States Patent Office Patented Aug. 26, 1953 thasaraywarrasants, 2. east one hydrogen atom attached thereto. That is, each of the tertiary halogens is capable of forming hydro 2,849,392 gen halide with a hydrogen atom attached to a different ANTIKNoCK COMPOSITIONS carbon atom which is in a position alpha to the halogen 5 bearing carbon. In this description the term tertiary car Raymond G. Lyben, Detroit, Mich., assigner to Ethy bon atom is defined as a carbon atom which has three Corporation, New York, N. Y., a corporation of Dear other carbon atoms attached thereto by single bonds. Ware Thus, the novel scavenging agents of this invention are No Drawing. Application May 31, 955 certain chlorohydrocarbons, bromohydrocarbons and Seria Rio. 52,284 chlorobromohydrocarbons. The halohydrocarbon scav enging agents of this invention can be derived from al 7 Clains. (C. 44-69) kanes, cycloalkanes, alkenes, cycloalkenes, and hydrocar bon-substituted derivatives thereof. The smallest hydro carbon radical which can provide a scavenger of this in This invention relates to improved antiknock composi s vention contains five carbon atoms; e. g., 1,2-dihalo-1,2- tions. These compositicins encompass antiknock fluids dimethylcyclopropane. In order to provide scavengers and leaded fuels. In particular, this invention relates to having the desirable volatility characteristics with respect a class of hydrocarbons having a particular molecular to the lead antiknock agent, I prefer to employ uniform structure for use as a scavenger with lead antiknock con ly stable scavengers having up to 20 carbon atoms. pounds. Thus, it will be seen that the carbon content of my scav With the discovery of the antiknock effectiveness of or engers ranges from 5 to 20 carbons per molecule. -

Liquid Biofuels: Substituting for Petroleum
Prospectus 77440.003.01 Liquid Biofuels: Substituting for Petroleum Prospectus Liquid Biofuels: Substituting for Petroleum April 2006 44 South Broadway, White Plains, New York 10601, USA Tel: +1 914 609 0300 Fax: +1 914 609 0399 Contents Section Page 1 Abstract........................................................................................................................ 1 2 Scope............................................................................................................................. 10 3 Approach ..................................................................................................................... 12 4 Contact Information ................................................................................................... 13 5 Authorization Form.................................................................................................... 14 6 Qualifications............................................................................................................... 16 6.1 GENERAL........................................................................................................ 16 6.2 SPECIFIC SINGLE-CLIENT EXPERIENCE RELEVANT TO BIOFUELS PRODUCTION AND USE............................................................................... 18 Cover photos - Courtesy of NREL Liquid Biofuels: Substituting for Petroleum i Q206_00806.001 Section 1 Abstract Nexant, Inc. (“Nexant”) has recently performed a number of analyses of technology and market prospects for biofuels, including studies on -

MTBE and New Hampshire's Waters
Remediating New Hampshire’s Groundwater A Comprehensive Study of MTBE in New Hampshire and Amicus Curiae Brief for State of New Hampshire v. Hess Corporation et al. A Major Qualifying Project Submitted to the faculty of Worcester Polytechnic Institute In partial fulfillment of the requirements for the Degree of Bachelor of Arts In Environmental & Sustainability Studies Susan Brennan Project Advisor: Robert Krueger March 2, 2013 2 Abstract New Hampshire’s groundwater has been contaminated by the gasoline additive methyl tertiary butyl ether (MTBE), a chemical that has dramatic repercussions on the environment. Once in the groundwater, MTBE can be difficult and expensive to treat and remove. New Hampshire hopes to hold the oil corporations that used MTBE in their gasoline accountable for the pollution caused by the additive through the Superior Court case State of New Hampshire v. Hess Corporation et al. This project both summarizes background research on MTBE and its implications on New Hampshire’s groundwater, as well as provides an amicus curiae brief in support of the state of New Hampshire in the case against the oil corporations. 3 Table of Contents Abstract ......................................................................................................................................................... 2 Technical Report What is MTBE? .............................................................................................................................................. 5 MTBE and United States Legislation ............................................................................................................ -

Common Myths and Misconceptions About the Behavior and Impact of MTBE Released from Petroleum Products
Transactions on Ecology and the Environment vol 65, © 2003 WIT Press, www.witpress.com, ISSN 1743-3541 Common myths and misconceptions about the behavior and impact of MTBE released from petroleum products R. E. woodward' & R. L. sloan2 '~ievvaEnvironmental Services, Inc., Houston, TX 2 Lyondell Chemical Company, Channelview, TX Abstract Auto fuel regulatory-mandates, to decrease the aromatic content of fuels and to reduce exhaust emissions, have led to the expanded use of additives to oxygenate auto fuels in the European Union (EU) and United States (US). The economical ether oxygenates, methyl tertiary butyl ether (MTBE) or ethyl tertiary butyl ether (ETBE) are frequently the oxygenate of choice because they deliver oxygen without increasing the Reid Vapor Pressure (RVP) or altering the fungible characteristics of autofuel. However, transport of auto fuels by common carriers that also transport heating oil and other heavier petroleum products has lead to the discovery of trace concentrations of auto fuel in many petroleum products. Subsequent leaks and spills during storage and handling of petroleum products result in the release of Auto fuel constituents to the environment. Critical review of 12 myths and misconceptions about MTBE in auto fuels reveals the concepts were conceived to rationalize early field observations andlor incomplete data sets. Closer scrutiny, in light of recent laboratory investigations, field data, case studies and world literature, indicates the myths are unsubstantiated misconceptions and assumptions about the behavior of ether oxygenates in the environment. Commonly held myths focus on four general areas of fuel and fuel oxygenates management: storageldispensing, hydrology, remediation, and health effects. Storageldispensing misconceptions address materials stability to ethers in fuel and the environmental forensics of fuel systems failure. -
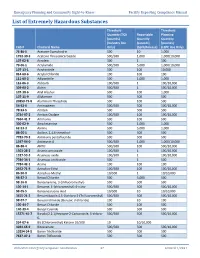
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Cumulative Cross Index to Iarc Monographs
RADIATION volume 100 D A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 2-9 June 2009 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns CUMULATIVE CROSS INDEX TO IARC MONOGRAPHS The volume, page and year of publication are given. References to corrigenda are given in parentheses. A A-α-C .............................................................40, 245 (1986); Suppl. 7, 56 (1987) Acenaphthene ........................................................................92, 35 (2010) Acepyrene ............................................................................92, 35 (2010) Acetaldehyde ........................36, 101 (1985) (corr. 42, 263); Suppl. 7, 77 (1987); 71, 319 (1999) Acetaldehyde associated with the consumption of alcoholic beverages ..............100E, 377 (2012) Acetaldehyde formylmethylhydrazone (see Gyromitrin) Acetamide .................................... 7, 197 (1974); Suppl. 7, 56, 389 (1987); 71, 1211 (1999) Acetaminophen (see Paracetamol) Aciclovir ..............................................................................76, 47 (2000) Acid mists (see Sulfuric acid and other strong inorganic acids, occupational exposures to mists and vapours from) Acridine orange ...................................................16, 145 (1978); Suppl. 7, 56 (1987) Acriflavinium chloride ..............................................13, 31 (1977); Suppl. 7, -

Tetraethyllead Is a Deadly Toxic Chemical Substance Giving Rise to Severe Psychotic Manifestations. for Its Excellent Properties
Industrial Health, 1986, 24, 139-150. Determination of Triethyllead, Diethyllead and Inorganic Lead in Urine by Atomic Absorption Spectrometry Fumio ARAI Department of Public Health St. Marianna University School of Medicine 2095 Sugao, Miyamae-ku, Kawasaki 213, Japan (Received March 10, 1986 and in revised form May 21, 1986) Abstract : A method was developed for the sequential extraction of tetraethyllead (Et4Pb), triethyllead (Et3Pb+), diethyllead (Et2Pb2+) and inorganic lead (Pb2+) from one urine sample with methyl isobutyl ketone and the subsequent sequential determination of the respective species of lead by flame and flameless atomic ab- sorption spectrometry. When 40 ml of a urine sample to which 2 ƒÊg of Pb of each of Et4Pb, Et3Pb+, Et2Pb2+ or Pb2+ had been experimentally added was assayed for the respective species of lead by flame atomic absorption spectrometry, ten repetitions of the assay gave a mean recovery rate of 98% for each of Et4Pb, Et3Pb+, and Et2Pb2+, and 99% for Pb2+, with a coefficient of variation of 2.0% for Et4Pb, 0.7% for Et3Pb+ and Pb2+, 2.6% for Et2Pb2+, and a detection limit of 4 ƒÊg of Pb/L for Et4Pb, 3 ƒÊg of Pb/L for Et3Pb+, and 5 ƒÊg of Pb/L for each of Et2Pb2+ and Pb2+. Examination of urine samples from a patient with tetraethyllead poisoning 22 days after exposure to the lead revealed that the total lead output was made up of about 51% Pb2+, about 43% Et2Pb2+, and about 6% Et3Pb+ but no Et4Pb. Ad- ministration of calcium ethylenediaminetetraacetic acid (Ca-EDTA) was followed by no increased urinary excretion of Et3Pb+ or Et2Pb2+. -

Catalytic Conversion of Syngas to Higher Alcohols Over Cu-Fe Based Catalysts
Mississippi State University Scholars Junction Theses and Dissertations Theses and Dissertations 1-1-2014 Catalytic Conversion of Syngas to Higher Alcohols over Cu-Fe Based Catalysts Yongwu Lu Follow this and additional works at: https://scholarsjunction.msstate.edu/td Recommended Citation Lu, Yongwu, "Catalytic Conversion of Syngas to Higher Alcohols over Cu-Fe Based Catalysts" (2014). Theses and Dissertations. 970. https://scholarsjunction.msstate.edu/td/970 This Dissertation - Open Access is brought to you for free and open access by the Theses and Dissertations at Scholars Junction. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholars Junction. For more information, please contact [email protected]. Automated Template C: Created by James Nail 2013V2.1 Catalytic conversion of syngas to higher alcohols over Cu-Fe based catalysts By Yongwu Lu A Dissertation Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biological Engineering in the Department of Agricultural and Biological Engineering Mississippi State, Mississippi December 2014 Copyright by Yongwu Lu 2014 Catalytic conversion of syngas to higher alcohols over Cu-Fe based catalysts By Yongwu Lu Approved: ____________________________________ Fei Yu (Major Professor) ____________________________________ Janice DuBien (Minor Professor) ____________________________________ Todd E. Mlsna (Committee Member) ____________________________________ -

Biogasoline Options for Conventional Spark-Ignition Cars
VTT WORKING PAPERS 187 Päivi Aakko-Saksa, Päivi Koponen, Johanna Kihlman, Matti Reinikainen, Eija Skyttä, Leena Rantanen-Kolehmainen & Ari Engman Biogasoline options for conventional spark-ignition cars ISBN 978-951-38-7529-9 (URL: http://www.vtt.fi/publications/index.jsp) ISSN 1459-7683 (URL: http://www.vtt.fi/publications/index.jsp) Copyright © VTT 2011 JULKAISIJA – UTGIVARE – PUBLISHER VTT, Vuorimiehentie 5, PL 1000, 02044 VTT puh. vaihde 020 722 111, faksi 020 722 4374 VTT, Bergsmansvägen 5, PB 1000, 02044 VTT tel. växel 020 722 111, fax 020 722 4374 VTT Technical Research Centre of Finland, Vuorimiehentie 5, P.O. Box 1000, FI-02044 VTT, Finland phone internat. +358 20 722 111, fax +358 20 722 4374 Series title, number and report code of publication VTT Working Papers 187 VTT-WORK-187 Author(s) Päivi Aakko-Saksa, Päivi Koponen, Johanna Kihlman, Matti Reinikainen, Eija Skyttä, Leena Rantanen-Kolehmainen & Ari Engman Title Biogasoline options for conventional spark-ignition cars Abstract The purpose of this study is to explore feasible gasoline biocomponents supplemen- tary to ethanol, and to assess their exhaust emissions performance. Although ethanol is the dominant liquid biofuel globally, technical restrictions limit its use in convention- al gasoline cars to 10–15 v/v% (bio-energy 7–10%). Since current conventional cars will continue to take the major share of gasoline car fleets for at least the next 10–20 years, it is necessary to establish and assess biocomponent options for them. Today, higher ethanol blending ratios are possible only with the use of flexible fuel vehicle (FFV) technology. The European Union requires renewable energy to have at least a 10% share of transport energy by 2020, and even higher shares are being attempted regionally. -

Utilization of Renewable Oxygenates As Gasoline Blend Components
Utilization of Renewable Oxygenates as Gasoline Blending Components Janet Yanowitz Ecoengineering, Inc. Earl Christensen and Robert L. McCormick Center for Transportation Technologies and Systems National Renewable Energy Laboratory NREL is a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency & Renewable Energy, operated by the Alliance for Sustainable Energy, LLC. Technical Report NREL/TP-5400-50791 August 2011 Contract No. DE-AC36-08GO28308 Utilization of Renewable Oxygenates as Gasoline Blending Components Janet Yanowitz Ecoengineering, Inc. Earl Christensen and Robert L. McCormick Center for Transportation Technologies and Systems National Renewable Energy Laboratory Prepared under Task No. FC08.9451 NREL is a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency & Renewable Energy, operated by the Alliance for Sustainable Energy, LLC. National Renewable Energy Laboratory Technical Report 1617 Cole Boulevard NREL/TP-5400-50791 Golden, Colorado 80401 August 2011 303-275-3000 • www.nrel.gov Contract No. DE-AC36-08GO28308 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof.