Abnormal High-Frequency Burst Firing of Cerebellar Neurons in Rapid-Onset Dystonia-Parkinsonism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Interactions Among Diameter, Myelination, and the Na/K Pump Affect Axonal Resilience to High-Frequency Spiking
Interactions among diameter, myelination, and the Na/K pump affect axonal resilience to high-frequency spiking Yunliang Zanga,b and Eve Mardera,b,1 aVolen National Center for Complex Systems, Brandeis University, Waltham, MA 02454; and bDepartment of Biology, Brandeis University, Waltham, MA 02454 Contributed by Eve Marder, June 22, 2021 (sent for review March 25, 2021; reviewed by Carmen C. Canavier and Steven A. Prescott) Axons reliably conduct action potentials between neurons and/or (16–21). It remains unknown how these directly correlated pro- other targets. Axons have widely variable diameters and can be cesses, fast spiking and slow Na+ removal, interact in the control myelinated or unmyelinated. Although the effect of these factors of reliable spike generation and propagation. Furthermore, it is on propagation speed is well studied, how they constrain axonal unclear whether reducing Na+-andK+-current overlap can con- resilience to high-frequency spiking is incompletely understood. sistently decrease the rate of Na+ influx and accordingly enhance Maximal firing frequencies range from ∼1Hzto>300 Hz across axonal reliability to propagate high-frequency spikes. Conse- neurons, but the process by which Na/K pumps counteract Na+ quently, by modeling the process of Na+ removal in unmyelinated influx is slow, and the extent to which slow Na+ removal is com- and myelinated axons, we systematically explored the effects of patible with high-frequency spiking is unclear. Modeling the pro- diameter, myelination, and Na/K pump density on spike propagation cess of Na+ removal shows that large-diameter axons are more reliability. resilient to high-frequency spikes than are small-diameter axons, because of their slow Na+ accumulation. -

Energy Expenditure Computation of a Single Bursting Neuron
Cognitive Neurodynamics (2019) 13:75–87 https://doi.org/10.1007/s11571-018-9503-3 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL ARTICLE Energy expenditure computation of a single bursting neuron 2 1,2 2 2 Fengyun Zhu • Rubin Wang • Xiaochuan Pan • Zhenyu Zhu Received: 17 October 2017 / Revised: 22 August 2018 / Accepted: 28 August 2018 / Published online: 3 September 2018 Ó The Author(s) 2018 Abstract Brief bursts of high-frequency spikes are a common firing pattern of neurons. The cellular mechanisms of bursting and its biological significance remain a matter of debate. Focusing on the energy aspect, this paper proposes a neural energy calculation method based on the Chay model of bursting. The flow of ions across the membrane of the bursting neuron with or without current stimulation and its power which contributes to the change of the transmembrane electrical potential energy are analyzed here in detail. We find that during the depolarization of spikes in bursting this power becomes negative, which was also discovered in previous research with another energy model. We also find that the neuron’s energy consumption during bursting is minimal. Especially in the spontaneous state without stimulation, the total energy con- sumption (2.152 9 10-7 J) during 30 s of bursting is very similar to the biological energy consumption (2.468 9 10-7 J) during the generation of a single action potential, as shown in Wang et al. (Neural Plast 2017, 2017a). Our results suggest that this property of low energy consumption could simply be the consequence of the biophysics of generating bursts, which is consistent with the principle of energy minimization. -

Sodium Pumps Mediate Activity-Dependent Changes in Mammalian Motor Networks
906 • The Journal of Neuroscience, January 25, 2017 • 37(4):906–921 Systems/Circuits Sodium Pumps Mediate Activity-Dependent Changes in Mammalian Motor Networks X Laurence D. Picton, XFilipe Nascimento, XMatthew J. Broadhead, XKeith T. Sillar, and XGareth B. Miles School of Psychology and Neuroscience, University of St Andrews, St Andrews KY16 9JP, United Kingdom Ubiquitously expressed sodium pumps are best known for maintaining the ionic gradients and resting membrane potential required for generating action potentials. However, activity- and state-dependent changes in pump activity can also influence neuronal firing and regulate rhythmic network output. Here we demonstrate that changes in sodium pump activity regulate locomotor networks in the spinal cord of neonatal mice. The sodium pump inhibitor, ouabain, increased the frequency and decreased the amplitude of drug-induced locomotor bursting, effects that were dependent on the presence of the neuromodulator dopamine. Conversely, activating the pump with the sodium ionophore monensin decreased burst frequency. When more “natural” locomotor output was evoked using dorsal-root stimulation, ouabain increased burst frequency and extended locomotor episode duration, whereas monensin slowed and shortened episodes. Decreasing the time between dorsal-root stimulation, and therefore interepisode interval, also shortened and slowed activity, suggesting that pump activity encodes information about past network output and contributes to feedforward control of subsequent locomotor bouts. Using whole-cell patch-clamp recordings from spinal motoneurons and interneurons, we describe a long-duration (ϳ60 s), activity-dependent, TTX- and ouabain-sensitive, hyperpolarization (ϳ5 mV), which is mediated by spike-dependent increases in pump activity. The duration of this dynamic pump potential is enhanced by dopamine. -

Characteristics of Hippocampal Primed Burst Potentiation in Vitro and in the Awake Rat
The Journal of Neuroscience, November 1988, 8(11): 4079-4088 Characteristics of Hippocampal Primed Burst Potentiation in vitro and in the Awake Rat D. M. Diamond, T. V. Dunwiddie, and G. M. Rose Medical Research Service, Veterans Administration Medical Center, and Department of Pharmacology, University of Colorado Health Sciences Center, Denver, Colorado 80220 A pattern of electrical stimulation based on 2 prominent (McNaughton, 1983; Lynch and Baudry, 1984; Farley and Al- physiological features of the hippocampus, complex spike kon, 1985; Byrne, 1987). Of these, a phenomenontermed long- discharge and theta rhythm, was used to induce lasting in- term potentiation (LTP) has received extensive attention be- creases in responses recorded in area CA1 of hippocampal causeit sharesa number of characteristicswith memory. For slices maintained in vitro and from the hippocampus of be- example, LTP has a rapid onset and long duration and is having rats. This effect, termed primed burst (PB) potentia- strengthenedby repetition (Barnes, 1979; Teyler and Discenna, tion, was elicited by as few as 3 stimuli delivered to the 1987). In addition, the decay of LTP is correlated with the time commissural/associational afferents to CAl. The patterns courseof behaviorally assessedforgetting (Barnes, 1979; Barnes of stimulus presentation consisted of a single priming pulse and McNaughton, 1985). The similarity of LTP and memory followed either 140 or 170 msec later by a high-frequency has fostered an extensive characterization of the conditions un- burst of 2-10 pulses; control stimulation composed of un- derlying the initiation of LTP, as well as its biochemical sub- primed high-frequency trains of up to 10 pulses had no en- strates (Lynch and Baudry, 1984; Wigstrijm and Gustafsson, during effect. -

The Logic Behind Neural Control of Breathing Pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C
www.nature.com/scientificreports OPEN The logic behind neural control of breathing pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C. A. Leite3 The respiratory rhythm generator is spectacular in its ability to support a wide range of activities and Received: 10 January 2019 adapt to changing environmental conditions, yet its operating mechanisms remain elusive. We show Accepted: 29 May 2019 how selective control of inspiration and expiration times can be achieved in a new representation of Published: xx xx xxxx the neural system (called a Boolean network). The new framework enables us to predict the behavior of neural networks based on properties of neurons, not their values. Hence, it reveals the logic behind the neural mechanisms that control the breathing pattern. Our network mimics many features seen in the respiratory network such as the transition from a 3-phase to 2-phase to 1-phase rhythm, providing novel insights and new testable predictions. Te mechanism for generating and controlling the breathing pattern by the respiratory neural circuit has been debated for some time1–6. In 1991, an area of the brainstem, the pre Bötzinger Complex (preBötC), was found essential for breathing7. An isolated single PreBötC neuron could generate tonic spiking (a non-interrupted sequence of action potentials), bursting (a repeating pattern that consists of a sequence of action potentials fol- lowed by a time interval with no action potentials) or silence (no action potentials)8. Tese signals are transmit- ted, through other populations of neurons, to spinal motor neurons that activate the respiratory muscle4,6. Te respiratory muscle contracts when it receives a sequence of action potentials from the motor neurons and relaxes when no action potentials arrive9. -
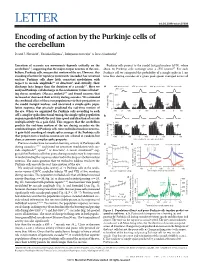
(2015) Encoding of Action by the Purkinje Cells of the Cerebellum
LETTER doi:10.1038/nature15693 Encoding of action by the Purkinje cells of the cerebellum David J. Herzfeld1, Yoshiko Kojima2, Robijanto Soetedjo2 & Reza Shadmehr1 Execution of accurate eye movements depends critically on the Purkinje cells project to the caudal fastigial nucleus (cFN), where cerebellum1–3, suggesting that the major output neurons of the cere- about 50 Purkinje cells converge onto a cFN neuron17. For each bellum, Purkinje cells, may predict motion of the eye. However, this Purkinje cell we computed the probability of a simple spike in 1-ms encoding of action for rapid eye movements (saccades) has remained time bins during saccades of a given peak speed, averaged across all unclear: Purkinje cells show little consistent modulation with respect to saccade amplitude4,5 or direction4, and critically, their discharge lasts longer than the duration of a saccade6,7.Herewe a 400° per s saccade 650° per s saccade 400° per s saccade 650° per s saccade analysed Purkinje-cell discharge in the oculomotor vermis of behav- 500° per s 8,9 200 ing rhesus monkeys (Macaca mulatta) and found neurons that N12 increased or decreased their activity during saccades. We estimated the combined effect of these two populations via their projections to 100 the caudal fastigial nucleus, and uncovered a simple-spike popu- lation response that precisely predicted the real-time motion of 0 the eye. When we organized the Purkinje cells according to each –1000 100 0 100 0 100 0 100 cell’s complex-spike directional tuning, the simple-spike population b 500° per s response predicted both the real-time speed and direction of saccade multiplicatively via a gain field. -

Comprehensive Analysis of the Expression of Sodium/Potassium-Atpase Α Subunits and Prognosis of Ovarian Serous Cystadenocarcinoma
Comprehensive Analysis of the Expression of Sodium/Potassium-ATPase α Subunits and Prognosis of Ovarian Serous Cystadenocarcinoma Wei Huang Tumor Hospital of Harbin Medical University Yongjian Zhang Tumor Hospital of Harbin Medical University Ye Xu Tumor Hospital of Harbin Medical University Shaoyou Yang Tumor Hospital of Harbin Medical University Bing Li Tumor Hospital of Harbin Medical University Lan Huang Tumor Hospital of Harbin Medical University Ge Lou ( [email protected] ) Tumor Hospital of Harbin Medical University https://orcid.org/0000-0001-6617-4482 Primary research Keywords: Adenosine triphosphate, ovary, cystadenocarcinoma, gynecology, gene expression Posted Date: June 22nd, 2020 DOI: https://doi.org/10.21203/rs.3.rs-23702/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on July 14th, 2020. See the published version at https://doi.org/10.1186/s12935-020-01414-5. Page 1/21 Abstract Background: Ovarian serous cystadenocarcinoma (OSC) is the most common and lethal gynecological cancer in women worldwide; however, biomarkers to diagnose and predict prognosis of OSC remain limited. Therefore, the present study aimed to investigate whether sodium/potassium adenosine triphosphate (Na+/K+-ATP)ase α-subunits (ATP1As) are helpful diagnostic and prognostic markers of OSC. Methods: Gene expression data (RNA-Seq) of 376 patients with OSC were downloaded from The Cancer Genome Atlas (TCGA) program database. Additional databases used in our analysis included the Gene Expression Omnibus, International Cancer Genome Consortium, Genotype–Tissue Expression, the Human Protein Atlas, cBioPortal for Cancer Genomics, and Cancer Cell Line Encyclopedia. -

A Recurrent De Novo Mutation in ATP1A3 Gene in a Mexican Patient with Alternating Hemiplegia of Childhood Detected by Massively Parallel Sequencing © Permanyer 2019
BoletínMédicodel HospitalInfantildeMéxico CLINICALCASE A recurrentde novo mutationinATP1A3 geneinaMexican patient withalternatinghemiplegiaofchildhooddetectedby massivelyparallelsequencing © Permanyer 2019 Carolina I. Galaz-Montoya1, Sofia Alcaraz-Estrada2, Leopoldo A. García-Montaño3, . Juan C. Zenteno3,4 and Raul E. Piña-Aguilar5* 1Private practice, Hermosillo, Sonora, Mexico; 2Division of Medical Genomics, Centro Médico Nacional 20 de Noviembre, ISSSTE, Mexico City, Mexico; 3Research Unit in Genetics, Instituto de Oftalmología Conde de Valencia, Mexico City, Mexico; 4Department of Biochemistry, Facultad de Medicina, Universidad Nacional Autónoma de Mexico, Mexico City, Mexico; 5School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, United Kingdom of the publisher Abstract Background: Pediatric movement disorders represent a diagnostic challenge for pediatricians and pediatric neurologists due to their high clinical heterogeneity and shared common features. Therefore, specific diagnoses require different approaches including metabolic work-up and specific tests for frequent genetic conditions. Alternating hemiplegia of childhood (AHC) is an ultra-rare pediatric movement disorder, characterized by paroxysmal alternating hemiplegia, dystonia, and seizure-like epi- sodes that can be misleading during the evaluation of a child with a movement disorder. Case report: We present a Mexican patient with abnormal movements referred to the Genetics clinic because of hyperammonemia and a possible organic acide- mia. Our assessment did not find clinical features compatible with an inborn error of metabolism. A massively parallel sequen- cing approach with targeted panel sequencing was used to get a final diagnosis. A missense variant c.2839G>A (p.Gly947Arg) located at exon 21 of ATP1A3 gene was demonstrated. This variant (rs398122887) has been previously reported as de novo producing alternating hemiplegia of childhood (AHC). -

Analysis of Complex Bursting in Cortical Pyramidal Neuron Modelsଝ
Neurocomputing 32}33 (2000) 181}187 Analysis of complex bursting in cortical pyramidal neuron modelsଝ Adam Kepecs*, Xiao-Jing Wang Volen Center for Complex Systems, Brandeis University, Waltham, MA 02254, USA Accepted 13 January 2000 Abstract Burst "ring is a prominent feature of cortical pyramidal cells and is thought to have signi"cant functional roles in reliable signaling and synaptic plasticity. Modeling studies have successfully elucidated possible biophysical mechanisms underlying complex bursting in pyr- amidal cells. Based on these results (Pinsky, Rinzel, J. Comput. Neurosci. 1 (1994) 39}60), we have built a simpli"ed two-compartment burst model. Using the fast- and slow-variable analysis method, we show that complex bursting is an instance of square-wave bursting, where the dendritic slow potassium conductance is the single slow variable. The coupling parameters between the two compartments change the topological class of bursting thereby altering the "ring patterns of the neuron. These results explain the diverse set of "ring patterns seen with di!erent dendritic morphologies (Mainen, Sejnowski, Nature 382 (1996) 363}366). ( 2000 Elsevier Science B.V. All rights reserved. Keywords: Phase-plane analysis; Complex burst; Bifurcation diagram 1. Introduction Pyramidal cells in many cortical areas "re stereotyped bursts of action potentials termed complex spikes or complex bursts. These bursts consist of 2}7 action poten- tials occurring in a &30 ms window. First observed in hippocampal single-unit extracellular recordings, these were termed &complex spikes' and were later identi"ed by intracellular techniques. Burst "ring is thought to play an important role in reliable signaling [5,15] and synaptic plasticity [2]. -

Epigenetic Mechanisms Are Involved in the Oncogenic Properties of ZNF518B in Colorectal Cancer
Epigenetic mechanisms are involved in the oncogenic properties of ZNF518B in colorectal cancer Francisco Gimeno-Valiente, Ángela L. Riffo-Campos, Luis Torres, Noelia Tarazona, Valentina Gambardella, Andrés Cervantes, Gerardo López-Rodas, Luis Franco and Josefa Castillo SUPPLEMENTARY METHODS 1. Selection of genomic sequences for ChIP analysis To select the sequences for ChIP analysis in the five putative target genes, namely, PADI3, ZDHHC2, RGS4, EFNA5 and KAT2B, the genomic region corresponding to the gene was downloaded from Ensembl. Then, zoom was applied to see in detail the promoter, enhancers and regulatory sequences. The details for HCT116 cells were then recovered and the target sequences for factor binding examined. Obviously, there are not data for ZNF518B, but special attention was paid to the target sequences of other zinc-finger containing factors. Finally, the regions that may putatively bind ZNF518B were selected and primers defining amplicons spanning such sequences were searched out. Supplementary Figure S3 gives the location of the amplicons used in each gene. 2. Obtaining the raw data and generating the BAM files for in silico analysis of the effects of EHMT2 and EZH2 silencing The data of siEZH2 (SRR6384524), siG9a (SRR6384526) and siNon-target (SRR6384521) in HCT116 cell line, were downloaded from SRA (Bioproject PRJNA422822, https://www.ncbi. nlm.nih.gov/bioproject/), using SRA-tolkit (https://ncbi.github.io/sra-tools/). All data correspond to RNAseq single end. doBasics = TRUE doAll = FALSE $ fastq-dump -I --split-files SRR6384524 Data quality was checked using the software fastqc (https://www.bioinformatics.babraham. ac.uk /projects/fastqc/). The first low quality removing nucleotides were removed using FASTX- Toolkit (http://hannonlab.cshl.edu/fastxtoolkit/). -

Supplementary Information Page
Supplementary information page Criteria for inclusion of patient variants in the analysis 1 Figure e-1 Alignment of the four human Na,K-ATPase alpha subunits 2-3 Figure e-2 Conformation differences when Na+ or K+ ions are bound 4 Figure e-3 Color-coded conservation scores for ATP1A1 generated by ConSurf 5 Figure e-4 Milder and more severe ATP1A3 pathogenic variants in the P domain are similar 6 Figure e-5 ATP1A2 and ATP1A3 variants in the S domain are also similar 7 Table e-1 Variants in ATP1A1 with references 8 Table e-2 Variants in ATP1A2 with references 9-13 Table e-3 Variants in ATP1A3 with references 14-19 Table e-4 Variants excluded from the analysis with references 20-21 Criteria for inclusion of patient variants in the analysis All included variants were missense, except that deletions of single amino acids were included without prejudice because several were recurrent in ATP1A2 and ATP1A3. The few more complicated genetic variants, splice site variants, and premature stops were not considered. Variants were considered pathogenic if: 1) identical variants arose independently in two or more unrelated patients/families with similar symptoms; 2) variants caused alternative amino acid changes in a single codon, with symptoms on the spectrum; 3) variants occurred at the equivalent position in more than one paralog, with symptoms typical of that paralog; or 4) there was laboratory evidence of impairment of Na,K-ATPase activity or biosynthesis. Single-family variants not meeting those criteria were considered probably pathogenic if 1) variants were proven to be de novo with DNA from both parents, or 2) pedigree data showed segregation with only affected members of the family, and 3) were considered possibly pathogenic when there was minimal or ambiguous pedigree data.