Newborn at Risk for Sepsis Executive Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibiotic Resistant Organisms Prevention and Control Guidelines for Healthcare Facilities
Antibiotic Resistant Organisms Prevention and Control Guidelines for Healthcare Facilities Reference Document for use by Health Care Organizations for Internal Policy/Protocol Development Revised from Provincial Infection Control Network Antibiotic Resistant Organisms Prevention and Control Guidelines 2008 Provincial Infection Control Network 2013 PICNet Antibiotic Resistant Organism (ARO) Guidelines Table of Contents Revisions Working Group ................................................................................................................... 3 Acronyms .......................................................................................................................................... 4 1 Introduction ................................................................................................................................ 5 2 Purpose ....................................................................................................................................... 5 3 Scope .......................................................................................................................................... 5 4 Literature Search Strategy ............................................................................................................ 6 5 Methods ...................................................................................................................................... 6 6 Background ................................................................................................................................ -

Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H
Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H. Long, MD, PhD,a,b Jennifer G. Fiore, MD,a,b Riaz Gillani, MD,a,b Laurie M. Douglass, MD,c Alan M. Fujii, MD,d Jodi D. Hoffman, MDe A 2-day old term male infant was found to be hypotonic and minimally abstract reactive during routine nursing care in the newborn nursery. At 40 hours of life, he was hypoglycemic and had intermittent desaturations to 70%. His mother had an unremarkable pregnancy and spontaneous vaginal delivery. The mother’s prenatal serology results were negative for infectious risk factors. Apgar scores were 9 at 1 and 5 minutes of life. On day 1 of life, he fed, stooled, and voided well. Our expert panel discusses the differential diagnosis of hypotonia in a neonate, offers diagnostic and management recommendations, and discusses the final diagnosis. DRS LONG, FIORE, AND GILLANI, birth weight was 3.4 kg (56th PEDIATRIC RESIDENTS percentile), length was 52 cm (87th aDepartment of Medicine, Boston Children’s Hospital, d e percentile), and head circumference Boston, Massachusetts; and Neonatology Section, Medical A 2-day old male infant born at Genetics Section, cDivision of Child Neurology, and 38 weeks and 4 days was found to be was 33 cm (12th percentile). His bDepartment of Pediatrics, Boston Medical Center, Boston, limp and minimally reactive during physical examination at birth was Massachusetts routine care in the newborn nursery. normal for gestational age, with Drs Long, Fiore, and Gillani conceptualized, drafted, Just 5 hours before, he had an appropriate neurologic, cardiac, and and edited the manuscript; Drs Douglass, Fujii, and appropriate neurologic status when respiratory components. -

Detecting Carbapenem-Resistant Acinetobacter Baumannii (CRAB) Carriage: Which Body Site Should Be Cultured?
Infection Control & Hospital Epidemiology (2020), 41, 965–967 doi:10.1017/ice.2020.197 Concise Communication Detecting carbapenem-resistant Acinetobacter baumannii (CRAB) carriage: Which body site should be cultured? Amir Nutman MD, MPH1,2 , Elizabeth Temkin DrPH1, Jonathan Lellouche PhD1, Debby Ben David MD1,2, David Schwartz PhD1 and Yehuda Carmeli MD, MPH1,2 1National Institute for Antibiotic Resistance and Infection Control, Ministry of Health, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel and 2Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel Abstract We compared the yield of culturing various body sites to detect carriage of carbapenem-resistant Acinetobacter baumannii (CRAB). Culturing the skin using a premoistened sponge, with overnight enrichment and plating on CHROMagar MDR Acinetobacter, had the highest yield: 92%. Skin is satisfactory as a single site for active surveillance of CRAB. (Received 8 January 2020; accepted 28 April 2020; electronically published 19 June 2020) Acinetobacter baumannii is a multidrug-resistant pathogen caus- The primary outcome was screening yield for each body site ing severe infections in hospitals and long-term care facilities. sampled. Because there is no gold standard for CRAB screening, Detecting carriers is a mainstay of controlling nosocomial spread a patient was defined as a CRAB carrier if a culture from any of of resistant organisms. Screening for carbapenem-resistant the sites sampled was positive. Acinetobacter baumannii (CRAB) has been recommended to control outbreaks, but no specific recommendations have been Specimen collection methods made regarding which body sites to culture.1 In 2007, we studied the yield of culturing the nose, throat, axilla, groin, rectum, open Buccal mucosa and rectal specimens were collected using swabs wounds, and tracheal aspirates, and no site or combination of (Amies agar gel transport swab; Copan Italia S.P.A., Brescia, sites had high enough sensitivity to be recommended for CRAB Italy). -

Hypotonia Surestep Product Catalog Page 29 in Step with Pediatric Hypotonia
SPECIAL EDUCATIONAL SERIES DIAGNOSTIC INSIGHTS ANALYZING GAIT CHANGES GROSS MOTOR SKILLS ORTHOTIC MANAGEMENT CLI N I CAL CASE STUDIES Sponsored by an educational grant from: In Step With Pediatric Hypotonia SureStep Product Catalog Page 29 In Step With Pediatric Hypotonia Contents VIEWPOINT FROM THE EDITOR: An Unexpected Path, Mobility and More an Invaluable Perspective At the most basic level, mobility is about get- PAGE 3 ting from point A to point B. But, for many children with hypotonia, it’s about so much 4 more. FEATURES It’s about independence. It’s about con- fidence. It’s about maintaining strength, fit- ness, and healthy bones. It’s about not being Understanding Hypotonia excluded from activities enjoyed by their PAGE 4 typically developing peers. And improved mobility may have even Gait: The Cornerstone more benefits in those children whose hy- potonia is associated with social and behav- of Intervention ioral developmental delays. New research PAGE 8 has identified an association between motor skills and sociobehavioral milestones in chil- 8 The Importance of Gross dren with autism spectrum disorder, who often present with hypotonia (see “The Im- Motor Skills portance of Gross Motor Skills,” page 12). PAGE 12 This suggests that early intervention to improve gross motor skills—including or- thotic devices and physical therapy—may Orthotic Solutions for also help certain children interact more Children with Hypotonia comfortably with others. That won’t come as PAGE 16 a surprise to the clinicians and parents who 12 have personally seen it happen. This special issue is filled with evidence- Orthotic Success Stories: based information and personal success sto- Four Cases in a Series ries illustrating how effective interventions can enhance mobility in children with hy- PAGE 20 potonia. -

Oregon Multidrug-Resistant Organism (MDRO) Toolkit
Ver. Oct 30, 2019 Oregon MDRO & C. difficile Toolkit OREGON MULTIDRUG-RESISTANT ORGANISM AND CLOSTRIDIOIDES DIFFICILE TOOLKIT Purpose Statement The purpose of this toolkit is to provide recommendations to Oregon healthcare facilities about strategies to prevent transmission of multidrug-resistant organisms (MDROs) and Clostridioides (formerly Clostridium) difficile during patient care. The toolkit recommendations provide general guidance and are not meant to replace facility-level policy or procedure. Local epidemiology as well as pertinent facility- or patient-level factors may affect the likelihood of MDRO transmission, and these factors should be taken into account when making decisions about transmission prevention strategies. The toolkit is intended to be a working document addressing high-impact organisms in Oregon hospitals. Given the continually evolving infection prevention and control landscape, including novel and emerging pathogens, this document will be updated as needed. Methods This toolkit was drafted by members of the Oregon Drug-Resistant Organism and Coordinated Regional Epidemiology (DROP-CRE) Network. To inform toolkit development, we convened a statewide Hospital Epidemiology Task Force to assist with two main objectives: 1) Optimize a practical approach to methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin- resistant enterococci (VRE) infection prevention and control; and 2) Establish statewide definitions for Gram-negative organisms in order to harmonize facility definitions, primarily for the purpose of uniform infection control practices. Over the course of 18 months, the Hospital Epidemiology Task Force reviewed existing drug- resistance definitions as published by collaboration of the European Centre for Disease Prevention and Control (eCDC) and the US Centers for Disease Control and Prevention (CDC).(1) In particular, the Task Force called attention to “possible extensively drug-resistant (XDR)” bacteria as those organisms harbor sufficiently broad drug resistance to substantially alter and limit treatment options. -

Screening for Asymptomatic Bacteriuria in Adults: an Updated Systematic Review for the U.S
Evidence Synthesis Number 183 Screening for Asymptomatic Bacteriuria in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA-290-2015-00007-I, Task Order No. 3 Prepared by: Kaiser Permanente Research Affiliates Evidence-based Practice Center Kaiser Permanente Center for Health Research Portland, OR Investigators: Jillian T. Henderson, PhD, MPH Elizabeth M. Webber, MS Sarah I. Bean, MPH AHRQ Publication No. 19-05252-EF-1 September 2019 This report is based on research conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (HHSA-290-2015-00007-I, Task Order No. 3). The findings and conclusions in this document are those of the authors, who are responsible for its contents, and do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients). -

(MRSA) Prevention Guidelines in Spinal Cord Injury Centers Using the PARIHS Framework: a Mixed Methods Study Salva N
Balbale et al. Implementation Science (2015) 10:130 DOI 10.1186/s13012-015-0318-x Implementation Science RESEARCH Open Access Evaluating implementation of methicillin- resistant Staphylococcus aureus (MRSA) prevention guidelines in spinal cord injury centers using the PARIHS framework: a mixed methods study Salva N. Balbale1,2,3†, Jennifer N. Hill1,2†, Marylou Guihan1,2,4†, Timothy P. Hogan5,6,7†, Kenzie A. Cameron8,9†, Barry Goldstein10,11† and Charlesnika T. Evans1,2,3,9* Abstract Background: To prevent methicillin-resistant Staphylococcus aureus (MRSA) in Spinal Cord Injury and Disorder (SCI/D) Centers, the “Guidelines for Implementation of MRSA Prevention Initiative in the Spinal Cord Injury Centers” were released in July 2008 in the Veterans Affairs (VA) Health Care System. The purpose of this study was to use the Promoting Action on Research Implementation in Health Systems (PARiHS) framework to evaluate the experiences of implementation of SCI/D MRSA prevention guidelines in VA SCI/D Centers approximately 2–3 years after the guidelines were released. Methods: Mixed methods were used across two phases in this study. The first phase included an anonymous, web-based cross-sectional survey administered toprovidersatall24VASCI/DCenters.Thesecondphaseincludedsemi-structured telephone interviews with providers at 9 SCI/D Centers. The PARiHS framework was used as the foundation of both the survey questions and semi-structured interview guide. Results: The survey was completed by 295 SCI/D providers (43.8 % response rate) from 22 of the 24 SCI/D Centers (91.7 % participation rate). Respondents included nurses (57.3 %), therapists (24.4 %), physicians (11.1 %), physician assistants (3.4 %), and other health care professionals (3.8 %). -
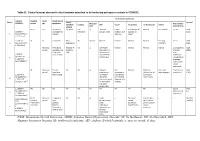
IUGR: Intrauterine Growth Restriction
Table S1. Clinical features observed in the 6 patients described so far harboring pathogenic variants in FOXRED1. Evolutionary symptoms Variants Prenatal Onset Onset clinical Patient Lactic/ Survival FOXRED1 period age symptoms Muscular Psychomotor Metabolic Epilepsy MRI Visual Respiratory Cardiovascular Others tone development acidosis IUGR 2m Hypotonia Yes (+++) Yes ↓ Normal Latent Bronchospasm Normal AEP normal IQ: 48 Alive c.920G>A Development refractary (2m,4y,7y3m) strabismus of episodes in (15y) 1 (p.Gly307Glu) / al delay right eye infant c.733+1G>A c.920G>A NI 4y Clumsiness With No Normal Normal Normal Normal Normal Learning IQ: 99 Alive 2 (p.Gly307Glu) / exercise (+) disorders (19y) c.733+1G>A - Neonatal Premature; No (only ↑ Yes ↓ Decreased Normal Normal Normal Normal Gradually loss Alive period Hypoglycemia lactate in attenuation in of motor (22y)) Congenital LCR) the putamen skills; c.694C>T lactic acidosis and cerebellar wheelchair; (p.Gln232X) / 3 atrophy (6y) no expressive c.1289A>G language; 18 (p.Asn430Ser) understands simple commands NI Neonatal Truncal Yes Yes ↓ Delayed Eye Normal Mild non- Persistent Psychomotor Alive period hypotonia myelination movements obstructive left hepatomegaly retardation (10y) c.1054 C>T Poor feeding ventricular have always ventricular (p.Arg352Trp) / dilatation; been roving hypertrophy 4 c.1054 C>T abnormal signal bilateral optic (p.Arg352Trp) 19 in the thalami atrophy and basal ganglia (8m) c.1308G>A ND ND ND ND Yes NA NA NA NA NA NA Severe Alive (p.Val421Met) / psychomotor (¿) 5 c.1308G>A retardation (p.Val421Met)20 IUGR; Neonatal Congenital Yes Yes ↓ Large -- Persistent Dilated right - - Death (3 c.612_615dupA Cerebral period lactic periventricular severe ventricle and months) CTG intraventric acidosis. -

Cerebral Hypotonia by Mihee Bay MD (Dr
Cerebral hypotonia By Mihee Bay MD (Dr. Bay of Kennedy Krieger Institute and Johns Hopkins School of Medicine has no relevant financial relationships to disclose.) Originally released July 12, 2006; last updated February 1, 2016; expires February 1, 2019 Introduction This article includes discussion of cerebral hypotonia, central hypotonia, essential hypotonia, benign congenital hypotonia, and floppy infant. The foregoing terms may include synonyms, similar disorders, variations in usage, and abbreviations. Overview Hypotonia is a clinical manifestation of numerous diseases affecting the central and/or peripheral motor nervous system. The key to accurate diagnosis involves integral steps of evaluation that include a detailed history, examination, and diagnostic tests. “Cerebral” (or central) hypotonia implies pathogenesis from abnormalities from the central nervous system, and related causal disorders include cerebral dysgenesis and genetic or metabolic disorders. Patients with central hypotonia generally have hypotonia without associated weakness, in contrast to the peripheral (lower motor neuron) causes, which typically produce both hypotonia and muscle weakness. Hypotonia is a clinical manifestation of over 500 genetic disorders; thus, a logical, stepwise approach to diagnosis is essential. With recent advances in the field of genetic testing, diagnostic yield will undoubtedly improve. There is no cure, but treatment includes supportive therapies, such as physical and occupational therapy, and diagnosis-specific management. Key points • Hypotonia is reduced tension or resistance of passive range of motion. • The first step in the evaluation of a child with hypotonia is localization to the central (“cerebral”) or peripheral nervous system, or both. • Central hypotonia is more likely to be noted axially with normal strength and hyperactive to normal deep tendon reflexes. -

Healthcare Associated Infections 2013 Report to the Wasington State
Healthcare Associated Infections 2013 Report to the Washington State Legislature April 2014 Division of Disease Control and Health Statistics Healthcare Associated Infections 2013 Report to the Washington State Legislature April 2014 DOH 420-113 For more information or additional copies of this report contact: Washington State Department of Health Division of Disease Control and Health Statistics 101 Israel Road S.E. P.O. Box 47811 Olympia WA 98504 Phone: 360-236-4202 Fax: 360-236-4245 John Wiesman, DrPH, MPH, CPH Secretary of Health Acknowledgements The Washington State Department of Health would like to thank past and current members of our Healthcare Associated Infections (HAI) Advisory Committee for their participation and expertise. Page i Table of Contents Executive Summary ...................................................................................................................... 1 I. Background ................................................................................................................................ 3 2013 Legislative Action on Healthcare Associated Infections ................................................................. 3 HAI Advisory Committee ......................................................................................................................... 4 National Activity ....................................................................................................................................... 4 The Washington State HAI Program ....................................................................................................... -

Mean Platelet Volume in Asymptomatic Chorioamnionitis-Exposed Infants
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2021;10(1):e100132 doi: 10.7363/100132 Received: 2019 Aug 22; revised: 2020 Jan 26; accepted: 2020 Feb 02; published online: 2020 Dec 28 Original article Mean platelet volume in asymptomatic chorioamnionitis- exposed infants. A retrospective case-control study Atef Alshafei, Moustafa Hassan, Yaser El saba, Anwar Khan, Mahmoud Ahmed Neonatology Section, Pediatric Department, Dubai Hospital, Dubai, UAE Abstract Introduction: Maternal chorioamnionitis (CA) is a serious condition causing several neonatal morbidities and long-term neurodevelopmental sequelae in exposed infants. Current guidelines still recommend admission, laboratory evaluation, and antibiotic administration to all CA-exposed infants. The incidence of early-onset neonatal sepsis (EOS) is currently low, owing to the routine intrapartum antibiotic administration to mothers identified to be at risk of developing CA. New diagnostic tools for early diagnosis of sepsis in apparently healthy infants exposed to maternal CA are needed. Previous studies showed that mean platelet volume (MPV) is evolving as a potential inflammatory marker of neonatal sepsis. We aimed to study whether MPV can be used as an adjuvant diagnostic tool for EOS in asymptomatic CA-exposed infants. Objective: To evaluate the role of MPV as an adjuvant biomarker of EOS in cases of asymptomatic CA-exposed infants. Design: Retrospective case-control study. Setting: A tertiary care Neonatal Intensive Care Unit (NICU). Patients: Asymptomatic CA-exposed infants 37-40 weeks of gestation admitted between May 2016 and April 2019 to the NICU of Dubai Hospital, UAE. Results: A total of 1,300 infants were admitted to NICU during the study period. -

Long-Term Outcome After Neonatal Meconium Obstruction
Long-term Outcome After Neonatal Meconium Obstruction Julie R. Fuchs, MD, and Jacob C. Langer, MD ABSTRACT. Objective. It is unclear whether children meconium ileus and those undergoing resection or enter- with cystic fibrosis (CF) who present with neonatal ostomy. Patients with meconium obstruction who do not meconium ileus have a different long-term outcome from have CF have an excellent long-term prognosis. This those presenting later in childhood with pulmonary com- information will be useful in counseling the families of plications or failure to thrive. We examined a cohort of infants presenting with neonatal meconium obstruction. patients with meconium ileus, and compared their long- Pediatrics 1998;101(4). URL: http://www.pediatrics.org/ term outcome with children who had CF without meco- cgi/content/full/101/4/e7; cystic fibrosis, meconium ileus, nium ileus and neonates who had meconium obstruction meconium plug syndrome. without CF (meconium plug syndrome). Study Design. Comparative study using retrospective and follow-up interview data. ABBREVIATION. CF, cystic fibrosis. Patients. Group 1 consisted of 35 surviving CF pa- tients who presented with meconium ileus between 1966 econium obstruction in the neonate is a and 1992. Two control groups were also studied: 35 age- spectrum of disease that includes meco- and sex-matched CF patients without meconium ileus 1 (group 2), and 12 infants presenting with meconium plug Mnium ileus and meconium plug syndrome. syndrome during the same time period (group 3). Meconium ileus is characterized by extremely viscid, Outcome Measures. Pulmonary, gastrointestinal, nu- protein-rich inspissated meconium causing terminal tritional, and functional status were reviewed, and sur- ileal obstruction, and accounts for approximately gical complications were recorded.