Natural Killer Cells Activated in a Human Mixed Lymphocyte Response Culture Identified by Expression of Leu-11 and Class Ii Histocompatibility Antigens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Killer Cells Associated with SARS-Cov-2 Viral RNA Shedding
Bao et al. Exp Hematol Oncol (2021) 10:5 https://doi.org/10.1186/s40164-021-00199-1 Experimental Hematology & Oncology LETTER TO THE EDITOR Open Access Natural killer cells associated with SARS-CoV-2 viral RNA shedding, antibody response and mortality in COVID-19 patients Changqian Bao1†, Xiandong Tao2,3†, Wei Cui4†, Yuanyuan Hao1, Shuaike Zheng5, Bin Yi2,3, Tiewen Pan2, Ken H. Young6* and Wenbin Qian1,7* Abstract Coronavirus disease 2019 (COVID-19) is a novel infectious viral disease caused by the severe acute respiratory syn- drome coronavirus 2 (SARS-CoV-2). Two consecutively negative SARS-CoV-2 viral RNA test ( interval 24 hours), improved respiratory symptoms and obvious absorption of infammation in pulmonary imaging are≥ the discharge criteria for COVID-19 patients. The clearance profle of viral RNA in the upper respiratory tract specimens, including nasopharyngeal swab and/or oropharyngeal swabs, is related to innate immune cells such as Natural Killer cells. A total of 168 patients were included for the study. In this cohort, non-severe and severe groups showed signifcant dif- ferences in white blood cells, neutrophils, lymphocytes, basophils and platelets counts, as well as in infection related parameters such as CRP and serum cytokine IL-6. For lymphocyte subsets tests at admission, the severe group dis- played signifcantly lower cell counts than the non-severe group. Higher counts of total T cells, CD4 T cells, CD8 T cells, and NK cells in peripheral blood showed a signifcant correlation with the shorter time taken to+ obtain the frst+ negative viral RNA test and frst positive IgM/ IgG antibody test. -

Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases
antibodies Review Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases Courtney A. Iberg and Daniel Hawiger * Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, Doisy Research Center, 1205 Carr Lane, St. Louis, MO 63104, USA; [email protected] * Correspondence: [email protected] Received: 31 March 2020; Accepted: 30 April 2020; Published: 15 June 2020 Abstract: The specific targeting of dendritic cells (DCs) using antigen-delivering antibodies has been established to be a highly efficient protocol for the induction of tolerance and protection from autoimmune processes in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), as well as in some other animal disease models. As the specific mechanisms of such induced tolerance are being investigated, the newly gained insights may also possibly help to design effective treatments for patients. Here we review approaches applied for the amelioration of autoimmunity in animal models based on antibody-mediated targeting of self-antigens to DCs. Further, we discuss relevant mechanisms of immunological tolerance that underlie such approaches, and we also offer some future perspectives for the application of similar methods in certain related disease settings such as transplantation. Keywords: dendritic cells; tolerance; antigen targeting; chimeric antibodies; autoimmunity; multiple sclerosis; diabetes 1. Introduction Over one hundred years ago, Paul Ehrlich coined the term “horror autotoxicus” to define an immune attack against an organism’s healthy tissues [1]. Since then, our knowledge of the complex mechanisms of the immune system as well as our understanding of the pathogenesis of specific autoimmune diseases have grown tremendously. -

Adaptive Immune Systems
Immunology 101 (for the Non-Immunologist) Abhinav Deol, MD Assistant Professor of Oncology Wayne State University/ Karmanos Cancer Institute, Detroit MI Presentation originally prepared and presented by Stephen Shiao MD, PhD Department of Radiation Oncology Cedars-Sinai Medical Center Disclosures Bristol-Myers Squibb – Contracted Research What is the immune system? A network of proteins, cells, tissues and organs all coordinated for one purpose: to defend one organism from another It is an infinitely adaptable system to combat the complex and endless variety of pathogens it must address Outline Structure of the immune system Anatomy of an immune response Role of the immune system in disease: infection, cancer and autoimmunity Organs of the Immune System Major organs of the immune system 1. Bone marrow – production of immune cells 2. Thymus – education of immune cells 3. Lymph Nodes – where an immune response is produced 4. Spleen – dual role for immune responses (especially antibody production) and cell recycling Origins of the Immune System B-Cell B-Cell Self-Renewing Common Progenitor Natural Killer Lymphoid Cell Progenitor Thymic T-Cell Selection Hematopoetic T-Cell Stem Cell Progenitor Dendritic Cell Myeloid Progenitor Granulocyte/M Macrophage onocyte Progenitor The Immune Response: The Art of War “Know your enemy and know yourself and you can fight a hundred battles without disaster.” -Sun Tzu, The Art of War Immunity: Two Systems and Their Key Players Adaptive Immunity Innate Immunity Dendritic cells (DC) B cells Phagocytes (Macrophages, Neutrophils) Natural Killer (NK) Cells T cells Dendritic Cells: “Commanders-in-Chief” • Function: Serve as the gateway between the innate and adaptive immune systems. -
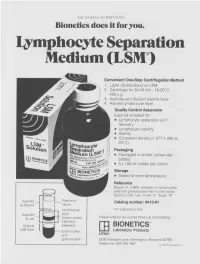
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Stress and the Immune System Tracy B
4 World Health • 47th Yeor, No. 2, Morch-Aprill994 Stress and the immune system Tracy B. Herbert any people have the effects of factors as diverse as experienced the examinations, bereavement, divorce, Mconnection between stress unemployment, mental arithmetic, and getting sick. Colds, influenza, and looking after a relative with herpes and allergies seem worse Alzheimer's di sease. In general, when we are severely stressed at these studies find that stress is work or in the home. Others are related to changes in both the never sick until they go on vacation numbers of white blood cells in (that is, after the stress is over), and circulation and the quantity of then they spend the whole time antibody in the blood. Moreover, fighting the virus. Because of stress is associated with changes in intrinsic connections like these, the functioning of immune cells. many researchers are today That is, there is a relatively large exploring whether (and how) stress decrease in both lymphocyte and illness are actually linked. One proliferation and natural killer cell specific focus of this research is to activity in individuals who have study the effects of stress on the experienced stress. There seems to immune systems; after all, if stress A lymphocyte: stress may weaken the capacity be some connection between the affects immunity, that would be one of lymphocytes to combat infection. duration of the stress and the amount way in which stress could contribute of immune change. For example, the to illness. longer the stress, the greater the The function of the immune proliferation"- by incubating these decrease in the number of specific system is to protect us from cells for several days with types of white blood cells. -

Association Between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center
biomedicines Article Association between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center Ji-Hoon Sim, Young-Jin Moon, Sung-Hoon Kim, Kyoung-Sun Kim , Ju-Seung Lee, Jun-Gol Song * and Gyu-Sam Hwang Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea; [email protected] (J.-H.S.); [email protected] (Y.-J.M.); [email protected] (S.-H.K.); [email protected] (K.-S.K.); [email protected] (J.-S.L.); [email protected] (G.-S.H.) * Correspondence: [email protected]; Tel.: +82-2-3010-3869 Abstract: Liver transplantation (LT) is closely associated with decreased immune function, a contrib- utor to herpes zoster (HZ). However, risk factors for HZ in living donor LT (LDLT) remain unknown. Neutrophil-lymphocyte ratio (NLR) and immune system function are reportedly correlated. This study investigated the association between NLR and HZ in 1688 patients who underwent LDLT between January 2010 and July 2020 and evaluated risk factors for HZ and postherpetic neuralgia (PHN). The predictive power of NLR was assessed through the concordance index and an integrated discrimination improvement (IDI) analysis. Of the total cohort, 138 (8.2%) had HZ. The incidence of HZ after LT was 11.2 per 1000 person-years and 0.1%, 1.3%, 2.9%, and 13.5% at 1, 3, 5, and 10 years, Citation: Sim, J.-H.; Moon, Y.-J.; Kim, respectively. In the Cox regression analysis, preoperative NLR was significantly associated with HZ S.-H.; Kim, K.-S.; Lee, J.-S.; Song, J.-G.; (adjusted hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.02–1.09; p = 0.005) and PHN (HR, Hwang, G.-S. -

Lymphocyte-Activation Gene 3 (LAG-3) Immune Pathway
Lymphocyte-Activation Gene 3 (LAG-3) About LAG-3 LAG-3 Lymphocyte-activation gene 3 (LAG-3) is an immune checkpoint receptor protein found on the cell surface of effector T cells and regulatory T cells (Tregs) and functions to control T cell response, activation and growth.1 TCR T cells are a type of white blood cell that are part of the immune system. Activation of cytotoxic T cells by antigens enables them to 1 kill unhealthy or foreign cells. Inactive T cell Antigen MHC Dendritic cell (APC) LAG-3 and LAG-3 and LAG-3 and Immune Function T Cell Exhaustion Cancer • After a T cell is activated to kill its • However, in certain situations where T • Because of its critical role in regulating target cell, LAG-3 expression is cells experience prolonged exposure to an exhaustion of cytotoxic T cells and Treg increased to turn off the immune antigen, such as cancer or chronic function, LAG-3 has become a target of response, so that the T cell does not go infection, the T cells become desensitized study in the cancer field. on to attack healthy cells.2 and lose their ability to activate and multiply in the presence of the antigen.4 • In cancer, LAG-3 expressing exhausted • Inhibition of the immune response is cytotoxic T cells and Tregs expressing accomplished through activation of • The desensitized T cell will also LAG-3 gather at tumor sites.5,6 the LAG-3 pathway, which can occur progressively fail to produce cytokines via binding of LAG-3 to a type of (proteins that assist in the immune • Preclinical studies suggest that inhibiting antigen-presenting complex called response) and kill the target cells.4 LAG-3 allows T cells to regain their MHC II. -

Understanding the Immune System: How It Works
Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute NIH Publication No. 03-5423 September 2003 www.niaid.nih.gov www.nci.nih.gov Contents 1 Introduction 2 Self and Nonself 3 The Structure of the Immune System 7 Immune Cells and Their Products 19 Mounting an Immune Response 24 Immunity: Natural and Acquired 28 Disorders of the Immune System 34 Immunology and Transplants 36 Immunity and Cancer 39 The Immune System and the Nervous System 40 Frontiers in Immunology 45 Summary 47 Glossary Introduction he immune system is a network of Tcells, tissues*, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes (germs)—tiny, infection-causing Bacteria: organisms such as bacteria, viruses, streptococci parasites, and fungi. Because the human body provides an ideal environment for many microbes, they try to break in. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them. Virus: When the immune system hits the wrong herpes virus target or is crippled, however, it can unleash a torrent of diseases, including allergy, arthritis, or AIDS. The immune system is amazingly complex. It can recognize and remember millions of Parasite: different enemies, and it can produce schistosome secretions and cells to match up with and wipe out each one of them. -

What You Need to Know About Innate Immunity
WhatWhat youyou needneed toto knowknow aboutabout innateinnate immunityimmunity JenniferJennifer KoKo MD,MD, PhDPhD ClevelandCleveland ClinicClinic Immunology/PathologyImmunology/Pathology andand LaboratoryLaboratory MedicineMedicine InnateInnate ImmunityImmunity FirstFirst lineline ofof defense,defense, immediateimmediate defensedefense DayDay toto dayday protectionprotection OnlyOnly whenwhen innateinnate defensedefense bypassed,bypassed, evadedevaded oror overwhelmedoverwhelmed isis adaptiveadaptive immunityimmunity requiredrequired NonNon--specificspecific RecognizeRecognize pathogenspathogens inin aa genericgeneric wayway DoesDoes notnot conferconfer longlong lastinglasting oror protectiveprotective immunityimmunity toto hosthost EvolutionarilyEvolutionarily older,older, foundfound inin primitiveprimitive organismsorganisms InnateInnate ImmunityImmunity andand InflammationInflammation 1)1) RespondRespond rapidlyrapidly toto tissuetissue damagedamage physicalphysical andand chemicalchemical barrierbarrier recruitmentrecruitment ofof immuneimmune cellscells toto sitesite ofof injuryinjury 2)2) LimitLimit spreadspread ofof infectioninfection identificationidentification andand removalremoval ofof foreignforeign substancessubstances activationactivation ofof thethe complementcomplement cascadecascade activationactivation ofof coagulationcoagulation cascadecascade 3)3) InitiateInitiate adaptiveadaptive immuneimmune responseresponse antigenantigen presentationpresentation andand cytokinecytokine productionproduction 4)4) -

Immunology 101
Immunology 101 Justin Kline, M.D. Assistant Professor of Medicine Section of Hematology/Oncology Committee on Immunology University of Chicago Medicine Disclosures • I served as a consultant on Advisory Boards for Merck and Seattle Genetics. • I will discuss non-FDA-approved therapies for cancer 2 Outline • Innate and adaptive immune systems – brief intro • How immune responses against cancer are generated • Cancer antigens in the era of cancer exome sequencing • Dendritic cells • T cells • Cancer immune evasion • Cancer immunotherapies – brief intro 3 The immune system • Evolved to provide protection against invasive pathogens • Consists of a variety of cells and proteins whose purpose is to generate immune responses against micro-organisms • The immune system is “educated” to attack foreign invaders, but at the same time, leave healthy, self-tissues unharmed • The immune system can sometimes recognize and kill cancer cells • 2 main branches • Innate immune system – Initial responders • Adaptive immune system – Tailored attack 4 The immune system – a division of labor Innate immune system • Initial recognition of non-self (i.e. infection, cancer) • Comprised of cells (granulocytes, monocytes, dendritic cells and NK cells) and proteins (complement) • Recognizes non-self via receptors that “see” microbial structures (cell wall components, DNA, RNA) • Pattern recognition receptors (PRRs) • Necessary for priming adaptive immune responses 5 The immune system – a division of labor Adaptive immune system • Provides nearly unlimited diversity of receptors to protect the host from infection • B cells and T cells • Have unique receptors generated during development • B cells produce antibodies which help fight infection • T cells patrol for infected or cancerous cells • Recognize “foreign” or abnormal proteins on the cell surface • 100,000,000 unique T cells are present in all of us • Retains “memory” against infections and in some cases, cancer. -

Early Lymphocyte Recovery Predicts Longer Survival After Autologous Peripheral Blood Stem Cell Transplantation in Multiple Myeloma
Bone Marrow Transplantation (2006) 37, 1037–1042 & 2006 Nature Publishing Group All rights reserved 0268-3369/06 $30.00 www.nature.com/bmt ORIGINAL ARTICLE Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma H Kim1, H-J Sohn2, S Kim2, J-S Lee2, W-K Kim2 and C Suh2 1Division of Hematology-Oncology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea and 2Division of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea To understand the prognostic value of lymphocyte Although the use of allogeneic hematopoietic stem cell recovery after autologous peripheral blood stem cell transplantation has been increasing, autologous stem cell transplantation (APBSCT), we performed a retrospective transplantation (ASCT) remains the standard therapeutic study of 59 newly diagnosed multiple myeloma (MM) modality for MM.1–3 The main drawback of ASCT is its patients who underwent frontline APBSCT. Conditioning high relapse rate. Because immune-mediated tumor eradi- regimens were melphalan 100 mg/m2 for 2 days. Follow- cation may lower the relapse rate associated with ASCT, ing APBSCT, all patients showed complete or partial there is increasing interest in immune reconstitution response. Median follow-up time was 29.57 months and following ASCT.4–9 In support of this, a relationship median recovery of absolute lymphocyte count (ALC) between lymphocyte recovery after ASCT and relapse rate X1000/mm3 was 23 days. Univariate analysis revealed or survival has been observed in many diseases, suggesting that significant predictors of overall survival (OS) that early lymphocyte recovery associated with immune included bone marrow (BM) plasma cells p40% at reconstitution can act against residual disease progres- diagnosis (P ¼ 0.0243) and recovery of ALC X1000/mm3 sion.10–17 by day þ 23 (P ¼ 0.0156). -

Could Antigen Presenting Cells Represent a Protective Element During SARS-Cov-2 Infection in Children?
pathogens Review Could Antigen Presenting Cells Represent a Protective Element during SARS-CoV-2 Infection in Children? Rita Lauro 1 , Natasha Irrera 1 , Ali H. Eid 2,3,* and Alessandra Bitto 1,* 1 Department of Clinical and Experimental Medicine, University of Messina, 98125 Messina, Italy; [email protected] (R.L.); [email protected] (N.I.) 2 Department of Basic Medical Sciences, College of Medicine, QU Health, Qatar University, P.O. Box 2713, Doha, Qatar 3 Biomedical and Pharmaceutical Research Unit, QU Health, Qatar University, P.O. Box 2713, Doha, Qatar * Correspondence: [email protected] (A.H.E.); [email protected] (A.B.) Abstract: Antigen Presenting Cells (APC) are immune cells that recognize, process, and present antigens to lymphocytes. APCs are among the earliest immune responders against an antigen. Thus, in patients with COVID-19, a disease caused by the newly reported SARS-CoV-2 virus, the role of APCs becomes increasingly important. In this paper, we dissect the role of these cells in the fight against SARS-CoV-2. Interestingly, this virus appears to cause a higher mortality among adults than children. This may suggest that the immune system, particularly APCs, of children may be different from that of adults, which may then explain differences in immune responses between these two populations, evident as different pathological outcome. However, the underlying molecular mechanisms that differentiate juvenile from other APCs are not well understood. Whether juvenile APCs are one reason why children are less susceptible to SARS-CoV-2 requires much attention. Citation: Lauro, R.; Irrera, N.; Eid, The goal of this review is to examine the role of APCs, both in adults and children.