Hexoprenaline Hydrochloride (BANM, Rinnm) ⊗ Onist
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
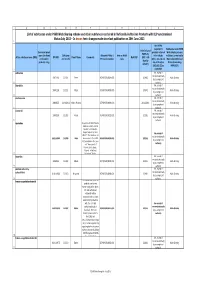
List of Substances Under PSUR WS Scheme and Other Substances
ABCDEFGHIJKL List of substances under PSUR Work Sharing scheme and other substances contained in Nationally Authorised Products with DLP synchronised Status July 2013 In brown font: changes made since last publication on 30th June 2013 1 Are PSURs required for Substances under PSUR Submission of Innovator brand products referred Work Sharing scheme PSURs by name (for fixed DLP (year Allocated PRMS / Note on PSUR to in Articles or Others (contained in Active substance name (INN) EU HBD Firm's Name Comments Next DLP (DLP + 90 combination and month) Procedure number cycle 10(1), 10a, 14 and Nationally Authorised days by products only) 16a of Directive Products including default) 2001/83/EC as MRP/DCP) 2 amended? sulbactam No, except if required nationally 19871116 201311 Pfizer AT/H/PSUR/0004/003 201402 Work Sharing by a competent 3 authority idarubicin No, except if required nationally 19891129 201311 Pfizer AT/H/PSUR/0005/003 201402 Work Sharing by a competent 4 authority dexibuprofen No, except if required nationally 20000725 31/08/2014 Gebro Pharma AT/H/PSUR/0009/003 29/11/2014 Work Sharing by a competent 5 authority nicorandil No, except if required nationally 19900209 201302 Merck AT/H/PSUR/0023/002 201305 Work Sharing by a competent 6 authority azelastine Request from MEDA Pharma GmbH & Co.KG to amend the DLP to 31/12/2014. Request agreed by the P- No, except if RMS AT. The substance has required nationally 20/11/1990 201309 Meda been moved to the EURD AT/H/PSUR/0038/001 201312 Work Sharing by a competent list as detailed in the -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Supplementary Information
1 SUPPLEMENTARY INFORMATION 2 ATIQ – further information 3 The Asthma Treatment Intrusiveness Questionnaire (ATIQ) scale was adapted from a scale originally 4 developed by Professor Horne to assess patients’ perceptions of the intrusiveness of antiretroviral 5 therapies (HAART; the HAART intrusiveness scale).1 This scale assesses convenience and the degree 6 to which the regimen is perceived by the patient to interfere with daily living, social life, etc. The 7 HAART intrusiveness scale has been applied to study differential effects of once- vs. twice-daily 8 antiretroviral regimens2 and might be usefully applied to identify patients who are most likely to 9 benefit from once-daily treatments. 10 11 References: 12 1. Newell, A., Mendes da Costa, S. & Horne, R. Assessing the psychological and therapy-related 13 barriers to optimal adherence: an observational study. Presented at the Sixth International 14 congress on Drug Therapy in HIV Infection, Glasgow, UK (2002). 15 2. Cooper, V., Horne, R., Gellaitry, G., Vrijens, B., Lange, A. C., Fisher, M. et al. The impact of once- 16 nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz- 17 based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr 53, 369–377 18 (2010). 19 1 20 Supplementary Table S1. Asthma medications, reported by participants at the time of survey Asthma medication n (%) Salbutamol 406 (40.2) Beclometasone 212 (21.0) Salmeterol plus fluticasone 209 (20.7) Salbutamol plus ipratropium 169 (16.7) Formoterol plus budesonide 166 -

GPCR/G Protein
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com GPCR/G Protein G Protein Coupled Receptors (GPCRs) perceive many extracellular signals and transduce them to heterotrimeric G proteins, which further transduce these signals intracellular to appropriate downstream effectors and thereby play an important role in various signaling pathways. G proteins are specialized proteins with the ability to bind the nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP). In unstimulated cells, the state of G alpha is defined by its interaction with GDP, G beta-gamma, and a GPCR. Upon receptor stimulation by a ligand, G alpha dissociates from the receptor and G beta-gamma, and GTP is exchanged for the bound GDP, which leads to G alpha activation. G alpha then goes on to activate other molecules in the cell. These effects include activating the MAPK and PI3K pathways, as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Most human GPCRs can be grouped into five main families named; Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin, forming the GRAFS classification system. A series of studies showed that aberrant GPCR Signaling including those for GPCR-PCa, PSGR2, CaSR, GPR30, and GPR39 are associated with tumorigenesis or metastasis, thus interfering with these receptors and their downstream targets might provide an opportunity for the development of new strategies for cancer diagnosis, prevention and treatment. At present, modulators of GPCRs form a key area for the pharmaceutical industry, representing approximately 27% of all FDA-approved drugs. References: [1] Moreira IS. Biochim Biophys Acta. 2014 Jan;1840(1):16-33. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Fenspiride Elisa Kit Instructions Product #108419 & 108416 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology FENSPIRIDE ELISA KIT INSTRUCTIONS PRODUCT #108419 & 108416 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Fenspiride and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Fenspiride ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug-enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Vr Meds Ex01 3B 0825S Coding Manual Supplement Page 1
vr_meds_ex01_3b_0825s Coding Manual Supplement MEDNAME OTHER_CODE ATC_CODE SYSTEM THER_GP PHRM_GP CHEM_GP SODIUM FLUORIDE A12CD01 A01AA01 A A01 A01A A01AA SODIUM MONOFLUOROPHOSPHATE A12CD02 A01AA02 A A01 A01A A01AA HYDROGEN PEROXIDE D08AX01 A01AB02 A A01 A01A A01AB HYDROGEN PEROXIDE S02AA06 A01AB02 A A01 A01A A01AB CHLORHEXIDINE B05CA02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D08AC02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D09AA12 A01AB03 A A01 A01A A01AB CHLORHEXIDINE R02AA05 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S01AX09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S02AA09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S03AA04 A01AB03 A A01 A01A A01AB AMPHOTERICIN B A07AA07 A01AB04 A A01 A01A A01AB AMPHOTERICIN B G01AA03 A01AB04 A A01 A01A A01AB AMPHOTERICIN B J02AA01 A01AB04 A A01 A01A A01AB POLYNOXYLIN D01AE05 A01AB05 A A01 A01A A01AB OXYQUINOLINE D08AH03 A01AB07 A A01 A01A A01AB OXYQUINOLINE G01AC30 A01AB07 A A01 A01A A01AB OXYQUINOLINE R02AA14 A01AB07 A A01 A01A A01AB NEOMYCIN A07AA01 A01AB08 A A01 A01A A01AB NEOMYCIN B05CA09 A01AB08 A A01 A01A A01AB NEOMYCIN D06AX04 A01AB08 A A01 A01A A01AB NEOMYCIN J01GB05 A01AB08 A A01 A01A A01AB NEOMYCIN R02AB01 A01AB08 A A01 A01A A01AB NEOMYCIN S01AA03 A01AB08 A A01 A01A A01AB NEOMYCIN S02AA07 A01AB08 A A01 A01A A01AB NEOMYCIN S03AA01 A01AB08 A A01 A01A A01AB MICONAZOLE A07AC01 A01AB09 A A01 A01A A01AB MICONAZOLE D01AC02 A01AB09 A A01 A01A A01AB MICONAZOLE G01AF04 A01AB09 A A01 A01A A01AB MICONAZOLE J02AB01 A01AB09 A A01 A01A A01AB MICONAZOLE S02AA13 A01AB09 A A01 A01A A01AB NATAMYCIN A07AA03 A01AB10 A A01 -

(12) Patent Application Publication (10) Pub. No.: US 2009/0047336A1 Yang Et Al
US 20090047336A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0047336A1 Yang et al. (43) Pub. Date: Feb. 19, 2009 (54) NOVEL FORMULATION OF DEHYDRATED Publication Classification LPID VESICLES FOR CONTROLLED (51) Int. Cl. RELEASE OF ACTIVE PHARMACEUTICAL A63/37 (2006.01) INGREDIENT VLANHALATION A6II 47/06 (2006.01) A 6LX 9/27 (2006.01) (75) Inventors: Zhijun Yang, Kowloon (CN); A6IR 9/14 (2006.01) Wenhua Huang, Kowloon (CN); A6IP II/00 (2006.01) Chi Sun Wong, Hong Kong (CN); A6IP II/06 (2006.01) Zhongzhen Zhao, Kowloon (CN) (52) U.S. Cl. .......... 424/450; 424/489: 514/653: 514/772 Correspondence Address: (57) ABSTRACT LEYDIG VOIT & MAYER, LTD A new formulation of dehydrated lipid vesicles employs a 700 THIRTEENTH ST. NW, SUITE 300 vesicle preserver and permits the control of release and deliv WASHINGTON, DC 20005-3960 (US) ery of active pharmaceutical ingredients into the respiratory system for treatment in particular of asthma. The typical (73) Assignee: HONG KONG BAPTIST formulation provides controlled release of the active pharma UNIVERSITY, Kowloon (CN) ceutical ingredient from 0% to 100% from 0 to 72 hours after inhalation, changes the systemic administration to topical (21) Appl. No.: 11/840,537 administration, allows prolonged therapeutic period for one administration, increased stability, with reduced dose, (22) Filed: Aug. 17, 2007 reduced systemic side effects, reduced toxicity. S Patent Application Publication US 2009/0047336A1 60 T. 50 40 30 20 O O Time (hours) --DOPC+1% gly cerin w8m DOTAP+1% glycerin FIG. 2 US 2009/0047336A1 Feb. 19, 2009 NOVEL FORMULATION OF DEHYDRATED blood pressure. -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] http://bmjopen.bmj.com/ on September 26, 2021 by guest. Protected copyright. BMJ Open BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from Treatment of stable chronic obstructive pulmonary disease: a protocol for a systematic review and evidence map Journal: BMJ Open ManuscriptFor ID peerbmjopen-2018-027935 review only Article Type: Protocol Date Submitted by the 15-Nov-2018 Author: Complete List of Authors: Dobler, Claudia; Mayo Clinic, Evidence-Based Practice Center, Robert D. -

Committed to Perform
Spine 8.5mm GLENMARK PHARMACEUTICALS LIMITED GLENMARK PHARMACEUTICALS Committed to perform Committed to perform ANNUAL REPORT 2015-16 REPORT ANNUAL www.glenmarkpharma.com ([email protected]) concept, content and design at ANNUAL REPORT 2015-16 JSNR_Glenmark AR 2016_Cover.indd 1 7/15/2016 3:31:04 PM Spine 8.5mm CONTENTS A TRIBUTE TO THE FOUNDER Glenmark at a Glance 02 Our Global Assets 04 Our Strategic Priorities 06 Chairman’s Message 08 Board of Directors 12 Committed to Perform 01 - Through Innovation 14 Corporate Overview - Through Global Expertise 20 - Through a Spirit of Excellence 24 Corporate Responsibility 26 Our People 30 Key Financials 32 Corporate Information 33 Management Discussion & Analysis 34 Prole of Directors 52 Board’s Report and Relevant Annexures 53 02 Report on Corporate Governance 90 Statutory Reports Standalone Auditor’s Report 105 Standalone Balance Sheet 110 Standalone Statement of Prot & Loss 111 Standalone Cash Flow Statement 112 Standalone Notes to Financial Statements 114 Founder: Late Mr. Gracias Saldanha Consolidated Auditor’s Report (IFRS) 148 Consolidated Statement of Financial Position (IFRS) 150 Consolidated Statement of Comprehensive Income (IFRS) 151 Consolidated Statement of Changes in Shareholder’s Equity 153 Achievement, Respect and Knowledge are the 03 (IFRS) Financial Statements Consolidated Statement of Cash Flow (IFRS) 154 values on which he built Glenmark. The seed of Notes to Consolidated Financial Statements (IFRS) 155 Consolidated Auditor’s Report (IGAAP) 195 our phenomenal growth story lies in the values Consolidated Balance Sheet (IGAAP) 198 instilled by our founder. And, it is these values Consolidated Statement of Prot & Loss (IGAAP) 199 Consolidated Cash Flow Statement (IGAAP) 200 which continue to guide the Company achieve Consolidated Notes to Financial Statements (IGAAP) 202 greater heights going ahead.