Larval Fish Dynamics in Coastal and Oceanic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synopsis Iconographique Des Otolithes De Quelques Espèces De Poissons Des Côtes Ouest Africaines
Synopsis iconographique des otolithes de quelques espèces de poissons des côtes ouest africaines Jan Veen et Kristiaan Hoedemakers VEDA consultancy Synopsis iconographique des otolithes de quelques espèces de poissons des côtes ouest africaines Jan Veen1, Kristiaan Hoedemakers2 1. VEDA consultancy, Wieselseweg 110, 7345 CC Wenum Wiesel, The Netherlands 2. Kristiaan Hoedemakers, Minervastraat 23, 2640 Mortsel, Belgium Wetlands International 2005 Copyright 2005 Wetlands International ISBN 9058829553 Cette publication doit être citée comme suit: Veen, J., Hoedemakers, K., 2005, Synopsis iconographique des otolithes de quelques espèces de poissons des côtes ouest africaines. Wageningen, The Netherlands. Publié par Wetlands International www.wetlands.org Dessins: Kristiaan Hoedemakers et Dirk Nolf. Tous droits réservés. Photos: Cindy van Damme, Alterra. Tous droits réservés. Texte: Jan Veen et Kristiaan Hoedemakers Lay-out: Kristiaan Hoedemakers Les données et désignations géographiques employées dans ce rapport n’impliquent en aucune manière une expression quelconque de l’opinion de la part de Wetlands International sur le statut légal d’un pays quel qu’il soit, d’une région ou d’un territoire, ou concernant la délimitation de ses limites ou frontières. Synopsis iconographique des otolithes de quelques espèces de poissons des côtes ouest africaines Organismes d’appui et de collaboration VEDA consultancy - research, advice and training in ecology and geography, The Netherlands VEDA consultancy Directorate General for International Co-operation, Ministry of Foreign Affairs, The Netherlands Directorate for Nature Management, Ministry of Agriculture, Nature and Food Quality, The Netherlands Financé par le Ministère de l’Agriculture, de la Nature et de la Qualité de l’Alimentation et le Ministère des Affaires Etrangères des Pays-Bas, dans le cadre du Programme Biodiversité de la Politique Internationale 2002-2006 des Pays-Bas. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Tfm Hanane El Yaagoubi
Máster Internacional en GESTIÓN PESQUERA SOSTENIBLE (7ª edición: 2017-2019) TESIS presentada y públicamente defendida para la obtención del título de MASTER OF SCIENCE HANANE EL YAAGOUBI Septiembre 2019 MASTERENGESTIÓNPESQUERASOSTENIBLE (7ªedición: 2017-2019) Spatiotemporal variation of fishery patterns, demographic indices and spatial distribution of European hake, Merluccius merluccius, in the GSA 01 and GSA03 Hanane EL YAAGOUBI TESIS PRESENTADA Y PUBLICAMENTE DEFENDIDA PARA LA OBTENCIÓN DEL TÍTULO DE MASTER OF SCIENCE EN GESTIÓN PESQUERA SOSTENIBLE Alicante a…09.de Septiembre de2019 ii Spatiotemporal variation of fishery patterns, demographic indices and spatial distribution of European hake, Merluccius merluccius, in the GSA 01 and GSA03 Hanane EL YAAGOUBI Trabajo realizado en el Centro Oceanográfico de Baleares (COB) del Instituto Español de Oceanografía (IEO), España, bajo la dirección del Dr.Manuel HIDALGO y la Dra. Pilar Hernández Y presentado como requisito parcial para la obtención del Diploma Master of Science en Gestión Pesquera Sostenible otorgado por la Universidad de Alicante a través de Facultad de Ciencias y el Centro Internacional de Altos Estudios Agronómicos Mediterráneos (CIHEAM) a través del Instituto Agronómico Mediterráneo de Zaragoza(IAMZ). V B Tutor y Tutora Autora Fdo:Dr.Manuel Hidalgo y Dra. Pilar Hernández... Fdo: Hanane El yaagoubi................. Alicante ,a 25 de Septiembre 2019 iii iv Spatiotemporal variation of fishery patterns, demographic indices and spatial distribution of European hake, Merluccius -

Geographic Structure of European Anchovy: a Nuclear-DNA Study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa
Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa To cite this version: Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa. Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research (JSR), Elsevier, 2008, 59, pp.269-278. 10.1016/j.seares.2008.03.001. hal-00553681 HAL Id: hal-00553681 https://hal.archives-ouvertes.fr/hal-00553681 Submitted on 8 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. To be cited as: Bouchenak-Khelladi Y., Durand J.-D., Magoulas A., Borsa P. 2008. – Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research 59, 269-278 [doi:10.1016/j.seares.2008.03.001] Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi a, Jean-Dominique Durand b, Antonios Magoulas c, Philippe Borsa d,* a Department of Botany, Trinity College, Dublin, Ireland b Institut de recherche pour le développement, Dakar, Senegal c Institute -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Worse Things Happen at Sea: the Welfare of Wild-Caught Fish
[ “One of the sayings of the Holy Prophet Muhammad(s) tells us: ‘If you must kill, kill without torture’” (Animals in Islam, 2010) Worse things happen at sea: the welfare of wild-caught fish Alison Mood fishcount.org.uk 2010 Acknowledgments Many thanks to Phil Brooke and Heather Pickett for reviewing this document. Phil also helped to devise the strategy presented in this report and wrote the final chapter. Cover photo credit: OAR/National Undersea Research Program (NURP). National Oceanic and Atmospheric Administration/Dept of Commerce. 1 Contents Executive summary 4 Section 1: Introduction to fish welfare in commercial fishing 10 10 1 Introduction 2 Scope of this report 12 3 Fish are sentient beings 14 4 Summary of key welfare issues in commercial fishing 24 Section 2: Major fishing methods and their impact on animal welfare 25 25 5 Introduction to animal welfare aspects of fish capture 6 Trawling 26 7 Purse seining 32 8 Gill nets, tangle nets and trammel nets 40 9 Rod & line and hand line fishing 44 10 Trolling 47 11 Pole & line fishing 49 12 Long line fishing 52 13 Trapping 55 14 Harpooning 57 15 Use of live bait fish in fish capture 58 16 Summary of improving welfare during capture & landing 60 Section 3: Welfare of fish after capture 66 66 17 Processing of fish alive on landing 18 Introducing humane slaughter for wild-catch fish 68 Section 4: Reducing welfare impact by reducing numbers 70 70 19 How many fish are caught each year? 20 Reducing suffering by reducing numbers caught 73 Section 5: Towards more humane fishing 81 81 21 Better welfare improves fish quality 22 Key roles for improving welfare of wild-caught fish 84 23 Strategies for improving welfare of wild-caught fish 105 Glossary 108 Worse things happen at sea: the welfare of wild-caught fish 2 References 114 Appendix A 125 fishcount.org.uk 3 Executive summary Executive Summary 1 Introduction Perhaps the most inhumane practice of all is the use of small bait fish that are impaled alive on There is increasing scientific acceptance that fish hooks, as bait for fish such as tuna. -

Fish, Crustaceans, Molluscs, Etc Capture Production by Species
488 Fish, crustaceans, molluscs, etc Capture production by species items Atlantic, Eastern Central C-34 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Atlantique, centre-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Atlántico, centro-oriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Tilapias nei Oreochromis (=Tilapia) spp 12 2 261 2 669 2 857 2 039 2 137 1 775 1 664 European eel Anguilla anguilla 22 9 ... 0 0 1 - - Shads nei Alosa spp 24 2 8 0 0 1 1 1 West African ilisha Ilisha africana 24 15 088 15 025 15 193 16 181 13 967 22 883 14 239 Mediterranean scaldfish Arnoglossus laterna 31 - - - - - - 57 Lefteye flounders nei Bothidae 31 46 ... 0 193 146 176 28 Common sole Solea solea 31 3 386 2 366 2 223 4 221 4 810 4 400 3 632 Sand sole Solea lascaris 31 ... ... 10 10 6 1 5 Wedge sole Dicologlossa cuneata 31 221 81 6 146 100 29 11 Soles nei Soleidae 31 5 264 6 167 8 273 9 313 10 575 6 830 8 290 Elongate tonguesole Symphurus ligulatus 31 - - - - - - 8 Tonguefishes Cynoglossidae 31 14 029 18 139 20 944 21 930 23 577 24 235 17 813 Megrim Lepidorhombus whiffiagonis 31 270 308 1 0 0 - 0 Turbot Psetta maxima 31 58 50 52 57 75 100 110 Turbots nei Scophthalmidae 31 ... ... ... ... ... ... 298 Citharids nei Citharidae 31 207 453 593 574 375 135 86 Spottail spiny turbot Psettodes belcheri 31 - 1 2 .. -

Merluccius Rafinesque, 1810 MERLU Merlu
click for previous page 327 Local Names : AUSTRALIA: Blue grenadier; GERMANY: Langschwanz-Seehecht; ITALY : Nasello azzurro; JAPAN : Hoki; NEW ZEALAND : Blue hake, Hoki, Whiptail; SPAIN : Merluza azul; USA : New Zealand whiptail. Literature : Armitage et al. (1981); Ayling & Cox (1982); Last et al. (1983). Remarks : Some specimens of Macruronus recently caught off western North Australia (18° S) might represent an undescribed species (N. Sinclair, pers. comm.). Merluccius Rafinesque, 1810 MERLU Merlu Genus with Reference : Merluccius Rafinesque, 1810:25. Type-species; Merluccius smiridus Rafinesque, 1810 (= Gadus merluccius Linnaeus, 1758) by monotypy. Diagnostic Features : Head large, about 1/3 to 1/4 of body length. Mouth large and oblique; maxillary reaching below middle of eye or behind it, almost half the length of head; lower jaw projecting below the upper; snout long and depressed, its length 1.3 to 3.2 times the eye diameter, its tip broad and rounded; eye large, its length 1/2 to 1/5 of upper jaw length; interorbital space broad, slightly elevated, its width 1.0 to 2.4 times the eye diameter; teeth in both jaws well developed, sharp, in two irregular rows; outer teeth fixed; inner ones larger and inwardly depressible; vomer with a biserial row of smaller teeth; no teeth on palatines; gill rakers well developed, varying in shape and number by species. Two separate dorsal fins, the first short, higher and triangular; the second long and partially divided by a notch at midlength; anal fin similar to second dorsal; pectoral fins long, slender and high in position, their relative length becoming smaller with growth; pelvic fins with 7 rays, placed in front of pectorals; caudal fin smaller than head and becoming progressively forked with growth; caudal skeleton possessing a set of X-Y bones. -

Proefschrift Muller
University of Groningen The commuting parent Mullers, Ralf Hubertus Elisabeth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2009 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Mullers, R. H. E. (2009). The commuting parent: Energetic constraints in a long distance forager, the Cape gannet. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 05-10-2021 The commuting parent Energetic constraints in a long distance forager, the Cape gannet The research reported in this thesis was supported by a grant from the Netherlands Foundation for the Advancement of Tropical Research (WOTRO) of the Netherlands Organisation for Scientific Research (NWO). -

Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights from Sardines and Anchovies and Lessons for Conservation
Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights From Sardines and Anchovies and Lessons for Conservation W. S. Grant and B. W. Bowen Most surveys of mitochondrial DNA (mtDNA) in marine ®shes reveal low levels of sequence divergence between haplotypes relative to the differentiation observed between sister taxa. It is unclear whether this pattern is due to rapid lineage sorting accelerated by sweepstakes recruitment, historical bottlenecks in population size, founder events, or natural selection, any of which could retard the accumulation of deep mtDNA lineages. Recent advances in paleoclimate research prompt a re- examination of oceanographic processes as a fundamental in¯uence on genetic diversity; evidence from ice cores and anaerobic marine sediments document strong regime shifts in the world's oceans in concert with periodic climatic changes. These changes in sea surface temperatures, current pathways, upwelling intensities, and retention eddies are likely harbingers of severe ¯uctuations in pop- ulation size or regional extinctions. Sardines (Sardina, Sardinops) and anchovies (Engraulis) are used to assess the consequences of such oceanographic process- es on marine ®sh intrageneric gene genealogies. Representatives of these two groups occur in temperate boundary currents on a global scale, and these regional populations are known to ¯uctuate markedly. Biogeographic and genetic data in- dicate that Sardinops has persisted for at least 20 million years, yet the mtDNA genealogy for this group coalesces in less than half a million years and points to a recent founding of populations around the rim of the Indian±Paci®c Ocean. Phy- logeographic analysis of Old World anchovies reveals a Pleistocene dispersal from the Paci®c to the Atlantic, almost certainly via southern Africa, followed by a very recent recolonization from Europe to southern Africa. -
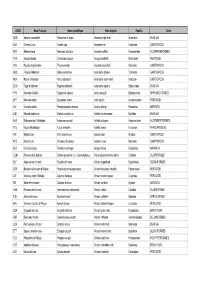
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

Merluccius Polli and M. Senegalensis (Merlucciidae) As First Records from the Canary Islands (North-Eastern Atlantic), SFI
Ichthyological note Merluccius polli and M. senegalensis (Merlucciidae) as first records from the Canary Islands (north-eastern Atlantic), SFI © with morphology data Submitted: 9 Sep. 2019 Accepted: 12 Dec. 2019 Editor: R. Causse by J. Gustavo GONZÁLEZ-LORENZO (1), Raül TRIAY-PORTELLA (2), José F. GONZÁLEZ-JIMÉNEZ (1), Pablo MARTÍN-SOSA (1), Sebastián JIMÉNEZ (1) & José A. GONZÁLEZ* (2) Résumé. – Premier signalement de Merluccius polli et M. senega- MATERIAL AND METHODS lensis (Merlucciidae) aux îles Canaries (Atlantique nord-est), et données morphologiques. The material studied was caught in waters of the Canary Islands (Fig. 1). La capture d’un individu de Merluccius polli et d’un autre de M. senegalensis représente le premier signalement pour ces espè- Both standard and ichthyological meristic/morphometric meas- ces tropicales aux îles Canaries. Deux hypothèses principales peu- urements (in mm) were made following Hubbs and Lagler (1958) vent être proposées pour expliquer la présence des deux espèces de and Lloris et al. (2005); SL, standard length; TL, total length. Fin- merlu noir dans les eaux des Canaries : des individus errants ou rays and vertebral counts with their distinguishing features were l’expansion de l’aire de répartition naturelle des zones voisines. Ce travail contribue également à la connaissance des caractéristiques obtained from radiographs, using X-ray equipment model Top 10 morphologiques et méristiques des deux espèces, y compris la for- with an 80 KHz high-frequency generator. mule vertébrale et ses caractéristiques distinctives. The voucher specimens were deposited in the collections of the Tenerife Museum of Natural History (TFMC). The present work Key words.