Global Seafood Production from Mariculture: Current Status, Trends and Its Future Under Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Highly Contiguous Genome Assembly of Pikeperch (Sander Lucioperca), an Emerging Aquaculture Species in Europe
G C A T T A C G G C A T genes Article The First Highly Contiguous Genome Assembly of Pikeperch (Sander lucioperca), an Emerging Aquaculture Species in Europe Julien Alban Nguinkal 1 , Ronald Marco Brunner 1,*, Marieke Verleih 1, Alexander Rebl 1 , Lidia de los Ríos-Pérez 2 , Nadine Schäfer 1, Frieder Hadlich 1, Marcus Stüeken 3, Dörte Wittenburg 2 and Tom Goldammer 1,* 1 Institute of Genome Biology, Leibniz Institute for Farm Animal Biology (FBN), 18196 Dummerstorf, Germany; [email protected] (J.A.N.); [email protected] (M.V.); [email protected] (A.R.); [email protected] (N.S.); [email protected] (F.H.) 2 Institute of Genetics and Biometry, Leibniz Institute for Farm Animal Biology (FBN), 18196 Dummerstorf, Germany; [email protected] (L.d.l.R.-P.); [email protected] (D.W.) 3 State Research Center of Agriculture and Fisheries M-V, 17194 Hohen Wangelin, Germany; [email protected] * Correspondence: [email protected] (R.M.B); [email protected] (T.G.); Tel.: +49-38208-68-708 (T.G.) Received: 23 July 2019; Accepted: 8 September 2019; Published: 13 September 2019 Abstract: The pikeperch (Sander lucioperca) is a fresh and brackish water Percid fish natively inhabiting the northern hemisphere. This species is emerging as a promising candidate for intensive aquaculture production in Europe. Specific traits like cannibalism, growth rate and meat quality require genomics based understanding, for an optimal husbandry and domestication process. Still, the aquaculture community is lacking an annotated genome sequence to facilitate genome-wide studies on pikeperch. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Endemik Beyşehir Kurbağası Pelophylax Caralitanus (Arıkan,1988) Populasyonlarında Comet Analizi Ile DNA Hasarın Değerlendirilmesi Uğur Cengiz Erişmiş
Instructions for Authors Scope of the Journal if there is more than one author at the end of the text. Su Ürünleri Dergisi (Ege Journal of Fisheries and Aquatic Sciences) is an open access, international, Hanging indent paragraph style should be used. The year of the reference should be in parentheses double blind peer-reviewed journal publishing original research articles, short communications, after the author name(s). The correct arrangement of the reference list elements should be in order technical notes, reports and reviews in all aspects of fisheries and aquatic sciences including biology, as “Author surname, first letter of the name(s). (publication date). Title of work. Publication data. DOI ecology, biogeography, inland, marine and crustacean aquaculture, fish nutrition, disease and Article title should be in sentence case and the journal title should be in title case. Journal titles in the treatment, capture fisheries, fishing technology, management and economics, seafood processing, Reference List must be italicized and spelled out fully; do not abbreviate titles (e.g., Ege Journal of chemistry, microbiology, algal biotechnology, protection of organisms living in marine, brackish and Fisheries and Aquatic Sciences, not Ege J Fish Aqua Sci). Article titles are not italicized. If the journal is freshwater habitats, pollution studies. paginated by issue the issue number should be in parentheses. Su Ürünleri Dergisi (EgeJFAS) is published quarterly (March, June, September and December) by Ege DOI information (if available) should be placed at the end of the reference as in the example. The DOI University Faculty of Fisheries since 1984. information for the reference list can be retrieved from CrossRef © Simple Text Query Form (http:// Submission of Manuscripts www.crossref.org/SimpleTextQuery/) by just pasting the reference list into the query box. -

Geographic Structure of European Anchovy: a Nuclear-DNA Study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa
Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa To cite this version: Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa. Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research (JSR), Elsevier, 2008, 59, pp.269-278. 10.1016/j.seares.2008.03.001. hal-00553681 HAL Id: hal-00553681 https://hal.archives-ouvertes.fr/hal-00553681 Submitted on 8 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. To be cited as: Bouchenak-Khelladi Y., Durand J.-D., Magoulas A., Borsa P. 2008. – Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research 59, 269-278 [doi:10.1016/j.seares.2008.03.001] Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi a, Jean-Dominique Durand b, Antonios Magoulas c, Philippe Borsa d,* a Department of Botany, Trinity College, Dublin, Ireland b Institut de recherche pour le développement, Dakar, Senegal c Institute -

Length-Weight Relationships of Marine Fish Collected from Around the British Isles
Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers This report should be cited as: Silva J. F., Ellis J. R. and Ayers R. A. 2013. Length-weight relationships of marine fish collected from around the British Isles. Sci. Ser. Tech. Rep., Cefas Lowestoft, 150: 109 pp. Additional copies can be obtained from Cefas by e-mailing a request to [email protected] or downloading from the Cefas website www.cefas.defra.gov.uk. © Crown copyright, 2013 This publication (excluding the logos) may be re-used free of charge in any format or medium for research for non-commercial purposes, private study or for internal circulation within an organisation. This is subject to it being re-used accurately and not used in a misleading context. The material must be acknowledged as Crown copyright and the title of the publication specified. This publication is also available at www.cefas.defra.gov.uk For any other use of this material please apply for a Click-Use Licence for core material at www.hmso.gov.uk/copyright/licences/ core/core_licence.htm, or by writing to: HMSO’s Licensing Division St Clements House 2-16 Colegate Norwich NR3 1BQ Fax: 01603 723000 E-mail: [email protected] 3 Contents Contents 1. Introduction 5 2. -

Worse Things Happen at Sea: the Welfare of Wild-Caught Fish
[ “One of the sayings of the Holy Prophet Muhammad(s) tells us: ‘If you must kill, kill without torture’” (Animals in Islam, 2010) Worse things happen at sea: the welfare of wild-caught fish Alison Mood fishcount.org.uk 2010 Acknowledgments Many thanks to Phil Brooke and Heather Pickett for reviewing this document. Phil also helped to devise the strategy presented in this report and wrote the final chapter. Cover photo credit: OAR/National Undersea Research Program (NURP). National Oceanic and Atmospheric Administration/Dept of Commerce. 1 Contents Executive summary 4 Section 1: Introduction to fish welfare in commercial fishing 10 10 1 Introduction 2 Scope of this report 12 3 Fish are sentient beings 14 4 Summary of key welfare issues in commercial fishing 24 Section 2: Major fishing methods and their impact on animal welfare 25 25 5 Introduction to animal welfare aspects of fish capture 6 Trawling 26 7 Purse seining 32 8 Gill nets, tangle nets and trammel nets 40 9 Rod & line and hand line fishing 44 10 Trolling 47 11 Pole & line fishing 49 12 Long line fishing 52 13 Trapping 55 14 Harpooning 57 15 Use of live bait fish in fish capture 58 16 Summary of improving welfare during capture & landing 60 Section 3: Welfare of fish after capture 66 66 17 Processing of fish alive on landing 18 Introducing humane slaughter for wild-catch fish 68 Section 4: Reducing welfare impact by reducing numbers 70 70 19 How many fish are caught each year? 20 Reducing suffering by reducing numbers caught 73 Section 5: Towards more humane fishing 81 81 21 Better welfare improves fish quality 22 Key roles for improving welfare of wild-caught fish 84 23 Strategies for improving welfare of wild-caught fish 105 Glossary 108 Worse things happen at sea: the welfare of wild-caught fish 2 References 114 Appendix A 125 fishcount.org.uk 3 Executive summary Executive Summary 1 Introduction Perhaps the most inhumane practice of all is the use of small bait fish that are impaled alive on There is increasing scientific acceptance that fish hooks, as bait for fish such as tuna. -

A Survey of Marine Bony Fishes of the Gaza Strip, Palestine
The Islamic University of Gaza الجــــــــــامعة اﻹســــــــــﻻميـة بغــــــــــــــــــزة Deanship of Research and Graduate Studies عمادة البحث العممي والدراسات العميا Faculty of Science كــــميـــــــــــــــــــــــــــــــة العـمـــــــــــــــــــــــــــــــــــــوم Biological Sciences Master Program ماجـســــــــتيـر العمــــــــــــــوم الحـياتيــــــــــة )Zoology) عمـــــــــــــــــــــــــــــــــــــــم حيـــــــــــــــــــــــــــــــــــــــوا A Survey of Marine Bony Fishes of the Gaza Strip, Palestine ِـخ ٌﻷؿّان اؼٌظٍّح اٌثذغٌح فً لطاع غؼج، فٍـطٍٓ By Huda E. Abu Amra B.Sc. Biology Supervised by Dr. Abdel Fattah N. Abd Rabou Associate Professor of Environmental Sciences A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biological Science (Zoology) August, 2018 إلــــــــــــــغاع أٔا اٌّىلغ أصٔاٖ ِمضَ اٌغؿاٌح اٌتً تذًّ اؼٌٕىاْ: A Survey of Marine Bony Fishes in the Gaza Strip, Palestine ِـخ ٌﻷؿّان اؼٌظٍّح اٌثذغٌح فً لطاع غؼج، فٍـطٍٓ أقش تأٌ يا اشرًهد عهّٛ ْزِ انشعانح إًَا ْٕ َراض جٓذ٘ انخاص، تاعرصُاء يا ذًد اﻹشاسج إنّٛ حٛصًا ٔسد، ٔأٌ ْزِ انشعانح ككم أٔ أ٘ جضء يُٓا نى ٚقذو يٍ قثم اٜخشٍٚ نُٛم دسجح أٔ نقة عهًٙ أٔ تحصٙ نذٖ أ٘ يؤعغح ذعهًٛٛح أٔ تحصٛح أخشٖ. Declaration I understand the nature of plagiarism, and I am aware of the University’s policy on this. The work provided in this thesis, unless otherwise referenced, is the researcher's own work, and has not been submitted by others elsewhere for any other degree or qualification. اعى انطانثح ْذٖ عٛذ عٛذ أتٕ عًشج :Student's name انرٕقٛع Signature: Huda انراسٚخ Date: 8-8-2018 I ٔتٍجح اٌذىُ ػٍى أطغودح ِاجـتٍغ II Abstract The East Mediterranean Sea is home to a wealth of marine resources including the bony fishes (Class Osteichthyes), which constitute a capital source of protein worldwide. -

Larval Fish Dynamics in Coastal and Oceanic
LARVAL FISH DYNAMICS IN COASTAL AND OCEANIC HABITATS IN THE CANARY CURRENT LARGE MARINE ECOSYSTEM (12 – 23°N) Dissertation with the aim of achieving a doctoral degree at the Faculty of Mathematics, Informatics and Natural Sciences Department of Biology of University Hamburg submitted by Maik Tiedemann M.Sc. Marine Biology B.Sc. Biology Hamburg, 2017 The present cumulative thesis is based on the scope on the bylaws of the Department of Biology's Doctoral Committee supplementing the Faculty of Mathematics, Informatics and Natural Sciences Doctoral Degree Regulations dated 1 December 2010 including adopted bylaws dated 23 February 2016. The content of chapter III may have changed in the process of publication and the submission of the present thesis. Please contact the principal author for citation purposes. Day of submission: 17. August 2017 Day of oral examination: 01. December 2017 The following evaluators recommend the admission of the dissertation: 1. Evaluator: Prof. Dr. Christian Möllmann Institute for Hydrobiology and Fisheries Science, Center for Earth System Research and Sustainability (CEN), Klima Campus, University of Hamburg, Grosse Elbstrasse 133, D-22767 Hamburg, Germany 2. Evaluator: Dr. Heino O. Fock Thünen-Institute (TI), Institute of Sea Fisheries, Federal Research Institute for Rural Areas, Forestry and Fisheries, Palmaille 9, 22767 Hamburg, Germany PREFACE The present dissertation cumulates the results of my doctoral project conducted from April 2013 to July 2017. My project was part of the tripartite German – French – African project “Ecosystem approach to the management of fisheries and the marine environment in West African waters” (AWA) funded by the German Federal Ministry of Education and Research (BMBF) under the grant number 01DG12073A. -

Proefschrift Muller
University of Groningen The commuting parent Mullers, Ralf Hubertus Elisabeth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2009 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Mullers, R. H. E. (2009). The commuting parent: Energetic constraints in a long distance forager, the Cape gannet. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 05-10-2021 The commuting parent Energetic constraints in a long distance forager, the Cape gannet The research reported in this thesis was supported by a grant from the Netherlands Foundation for the Advancement of Tropical Research (WOTRO) of the Netherlands Organisation for Scientific Research (NWO). -

Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights from Sardines and Anchovies and Lessons for Conservation
Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights From Sardines and Anchovies and Lessons for Conservation W. S. Grant and B. W. Bowen Most surveys of mitochondrial DNA (mtDNA) in marine ®shes reveal low levels of sequence divergence between haplotypes relative to the differentiation observed between sister taxa. It is unclear whether this pattern is due to rapid lineage sorting accelerated by sweepstakes recruitment, historical bottlenecks in population size, founder events, or natural selection, any of which could retard the accumulation of deep mtDNA lineages. Recent advances in paleoclimate research prompt a re- examination of oceanographic processes as a fundamental in¯uence on genetic diversity; evidence from ice cores and anaerobic marine sediments document strong regime shifts in the world's oceans in concert with periodic climatic changes. These changes in sea surface temperatures, current pathways, upwelling intensities, and retention eddies are likely harbingers of severe ¯uctuations in pop- ulation size or regional extinctions. Sardines (Sardina, Sardinops) and anchovies (Engraulis) are used to assess the consequences of such oceanographic process- es on marine ®sh intrageneric gene genealogies. Representatives of these two groups occur in temperate boundary currents on a global scale, and these regional populations are known to ¯uctuate markedly. Biogeographic and genetic data in- dicate that Sardinops has persisted for at least 20 million years, yet the mtDNA genealogy for this group coalesces in less than half a million years and points to a recent founding of populations around the rim of the Indian±Paci®c Ocean. Phy- logeographic analysis of Old World anchovies reveals a Pleistocene dispersal from the Paci®c to the Atlantic, almost certainly via southern Africa, followed by a very recent recolonization from Europe to southern Africa. -
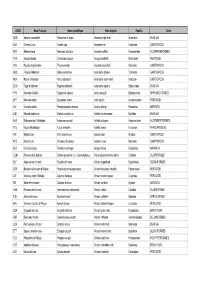
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

Review of the State of World Marine Fishery Resources
ISSN 2070-7010 569 FAO FISHERIES AND AQUACULTURE TECHNICAL PAPER 569 Review of the state of world marine fishery resources Review of the state world marine fishery resources This publication presents an updated assessment and review of the current status of the world’s marine fishery resources. It summarizes the information available for each FAO Statistical Areas; discusses the major trends and changes that have occurred with the main fishery resources exploited in each area; and reviews the stock assessment work undertaken in support of fisheries management in each region. The review is based mainly on official catch statistics up until 2009 and relevant stock assessment and other complementary information available until 2010. It aims to provide the FAO Committee on Fisheries and, more generally, policy-makers, civil society, fishers and managers of world fishery resources with a comprehensive, objective and global review of the state of the living marine resources. ISBN 978-92-5-107023-9 ISSN 2070-7010 FAO 9 789251 070239 I2389E/1/12.11 Cover illustration: Emanuela D’Antoni FAO FISHERIES AND Review of the state AQUACULTURE TECHNICAL of world marine PAPER fishery resources 569 Marine and Inland Fisheries Service Fisheries and Aquaculture Resources Use and Conservation Division FAO Fisheries and Aquaculture Department FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.