Multidisciplinary Investigation Into Stock Structure of Small Pelagic Fishes in Southern Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geographic Structure of European Anchovy: a Nuclear-DNA Study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa
Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa To cite this version: Yanis Bouchenak-Khelladi, Jean-Dominique Durand, Antonios Magoulas, Philippe Borsa. Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research (JSR), Elsevier, 2008, 59, pp.269-278. 10.1016/j.seares.2008.03.001. hal-00553681 HAL Id: hal-00553681 https://hal.archives-ouvertes.fr/hal-00553681 Submitted on 8 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. To be cited as: Bouchenak-Khelladi Y., Durand J.-D., Magoulas A., Borsa P. 2008. – Geographic structure of European anchovy: a nuclear-DNA study. Journal of Sea Research 59, 269-278 [doi:10.1016/j.seares.2008.03.001] Geographic structure of European anchovy: a nuclear-DNA study Yanis Bouchenak-Khelladi a, Jean-Dominique Durand b, Antonios Magoulas c, Philippe Borsa d,* a Department of Botany, Trinity College, Dublin, Ireland b Institut de recherche pour le développement, Dakar, Senegal c Institute -

Worse Things Happen at Sea: the Welfare of Wild-Caught Fish
[ “One of the sayings of the Holy Prophet Muhammad(s) tells us: ‘If you must kill, kill without torture’” (Animals in Islam, 2010) Worse things happen at sea: the welfare of wild-caught fish Alison Mood fishcount.org.uk 2010 Acknowledgments Many thanks to Phil Brooke and Heather Pickett for reviewing this document. Phil also helped to devise the strategy presented in this report and wrote the final chapter. Cover photo credit: OAR/National Undersea Research Program (NURP). National Oceanic and Atmospheric Administration/Dept of Commerce. 1 Contents Executive summary 4 Section 1: Introduction to fish welfare in commercial fishing 10 10 1 Introduction 2 Scope of this report 12 3 Fish are sentient beings 14 4 Summary of key welfare issues in commercial fishing 24 Section 2: Major fishing methods and their impact on animal welfare 25 25 5 Introduction to animal welfare aspects of fish capture 6 Trawling 26 7 Purse seining 32 8 Gill nets, tangle nets and trammel nets 40 9 Rod & line and hand line fishing 44 10 Trolling 47 11 Pole & line fishing 49 12 Long line fishing 52 13 Trapping 55 14 Harpooning 57 15 Use of live bait fish in fish capture 58 16 Summary of improving welfare during capture & landing 60 Section 3: Welfare of fish after capture 66 66 17 Processing of fish alive on landing 18 Introducing humane slaughter for wild-catch fish 68 Section 4: Reducing welfare impact by reducing numbers 70 70 19 How many fish are caught each year? 20 Reducing suffering by reducing numbers caught 73 Section 5: Towards more humane fishing 81 81 21 Better welfare improves fish quality 22 Key roles for improving welfare of wild-caught fish 84 23 Strategies for improving welfare of wild-caught fish 105 Glossary 108 Worse things happen at sea: the welfare of wild-caught fish 2 References 114 Appendix A 125 fishcount.org.uk 3 Executive summary Executive Summary 1 Introduction Perhaps the most inhumane practice of all is the use of small bait fish that are impaled alive on There is increasing scientific acceptance that fish hooks, as bait for fish such as tuna. -

Larval Fish Dynamics in Coastal and Oceanic
LARVAL FISH DYNAMICS IN COASTAL AND OCEANIC HABITATS IN THE CANARY CURRENT LARGE MARINE ECOSYSTEM (12 – 23°N) Dissertation with the aim of achieving a doctoral degree at the Faculty of Mathematics, Informatics and Natural Sciences Department of Biology of University Hamburg submitted by Maik Tiedemann M.Sc. Marine Biology B.Sc. Biology Hamburg, 2017 The present cumulative thesis is based on the scope on the bylaws of the Department of Biology's Doctoral Committee supplementing the Faculty of Mathematics, Informatics and Natural Sciences Doctoral Degree Regulations dated 1 December 2010 including adopted bylaws dated 23 February 2016. The content of chapter III may have changed in the process of publication and the submission of the present thesis. Please contact the principal author for citation purposes. Day of submission: 17. August 2017 Day of oral examination: 01. December 2017 The following evaluators recommend the admission of the dissertation: 1. Evaluator: Prof. Dr. Christian Möllmann Institute for Hydrobiology and Fisheries Science, Center for Earth System Research and Sustainability (CEN), Klima Campus, University of Hamburg, Grosse Elbstrasse 133, D-22767 Hamburg, Germany 2. Evaluator: Dr. Heino O. Fock Thünen-Institute (TI), Institute of Sea Fisheries, Federal Research Institute for Rural Areas, Forestry and Fisheries, Palmaille 9, 22767 Hamburg, Germany PREFACE The present dissertation cumulates the results of my doctoral project conducted from April 2013 to July 2017. My project was part of the tripartite German – French – African project “Ecosystem approach to the management of fisheries and the marine environment in West African waters” (AWA) funded by the German Federal Ministry of Education and Research (BMBF) under the grant number 01DG12073A. -

Proefschrift Muller
University of Groningen The commuting parent Mullers, Ralf Hubertus Elisabeth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2009 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Mullers, R. H. E. (2009). The commuting parent: Energetic constraints in a long distance forager, the Cape gannet. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 05-10-2021 The commuting parent Energetic constraints in a long distance forager, the Cape gannet The research reported in this thesis was supported by a grant from the Netherlands Foundation for the Advancement of Tropical Research (WOTRO) of the Netherlands Organisation for Scientific Research (NWO). -

Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights from Sardines and Anchovies and Lessons for Conservation
Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights From Sardines and Anchovies and Lessons for Conservation W. S. Grant and B. W. Bowen Most surveys of mitochondrial DNA (mtDNA) in marine ®shes reveal low levels of sequence divergence between haplotypes relative to the differentiation observed between sister taxa. It is unclear whether this pattern is due to rapid lineage sorting accelerated by sweepstakes recruitment, historical bottlenecks in population size, founder events, or natural selection, any of which could retard the accumulation of deep mtDNA lineages. Recent advances in paleoclimate research prompt a re- examination of oceanographic processes as a fundamental in¯uence on genetic diversity; evidence from ice cores and anaerobic marine sediments document strong regime shifts in the world's oceans in concert with periodic climatic changes. These changes in sea surface temperatures, current pathways, upwelling intensities, and retention eddies are likely harbingers of severe ¯uctuations in pop- ulation size or regional extinctions. Sardines (Sardina, Sardinops) and anchovies (Engraulis) are used to assess the consequences of such oceanographic process- es on marine ®sh intrageneric gene genealogies. Representatives of these two groups occur in temperate boundary currents on a global scale, and these regional populations are known to ¯uctuate markedly. Biogeographic and genetic data in- dicate that Sardinops has persisted for at least 20 million years, yet the mtDNA genealogy for this group coalesces in less than half a million years and points to a recent founding of populations around the rim of the Indian±Paci®c Ocean. Phy- logeographic analysis of Old World anchovies reveals a Pleistocene dispersal from the Paci®c to the Atlantic, almost certainly via southern Africa, followed by a very recent recolonization from Europe to southern Africa. -
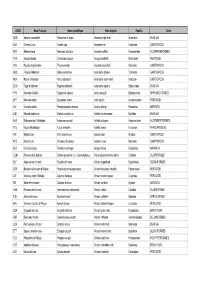
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

Global Seafood Production from Mariculture: Current Status, Trends and Its Future Under Climate Change
GLOBAL SEAFOOD PRODUCTION FROM MARICULTURE: CURRENT STATUS, TRENDS AND ITS FUTURE UNDER CLIMATE CHANGE by Muhammed Alolade Oyinlola B. AQFM., University of Agriculture, Abeokuta, Nigeria, 2010 M.Sc., Universität Bremen, Germany, 2014 A DISSERTATION SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate and Postdoctoral Studies (Zoology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2019 © Muhammed Alolade Oyinlola, 2019 The following individuals certify that they have read, and recommend to the Faculty of Graduate and Postdoctoral Studies for acceptance, the dissertation entitled: Global seafood production from mariculture: current status, trends and its future under climate change submitted by Muhammed Alolade Oyinlola in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Zoology Examining Committee: Dr. William Wai Lung Cheung Supervisor Dr. Daniel Pauly Supervisory Committee Member Dr. Evgeny Pakhomov University Examiner Dr. Xiaonan Lu University Examiner Additional Supervisory Committee Members: Dr. Rashid Sumaila Supervisory Committee Member Dr. Max Troell Supervisory Committee Member ii Abstract Mariculture is growing rapidly over the last three decades at an average rate of about 3.7% per year from 2001 to 2010. However, questions about mariculture sustainable development are uncertain because of diverse environmental challenges and concerns that the sector faces. Changing ocean conditions such as temperature, acidity, oxygen level and primary production can affect mariculture production, directly and indirectly, particularly the open and semi-open ocean farming operations. This dissertation aims to understand climate change impact on future seafood production from mariculture. Firstly, I update the existing Global Mariculture Database (GMD) with recent mariculture production and create a farm-gate price database to match the production data. -

Review of the State of World Marine Fishery Resources
ISSN 2070-7010 569 FAO FISHERIES AND AQUACULTURE TECHNICAL PAPER 569 Review of the state of world marine fishery resources Review of the state world marine fishery resources This publication presents an updated assessment and review of the current status of the world’s marine fishery resources. It summarizes the information available for each FAO Statistical Areas; discusses the major trends and changes that have occurred with the main fishery resources exploited in each area; and reviews the stock assessment work undertaken in support of fisheries management in each region. The review is based mainly on official catch statistics up until 2009 and relevant stock assessment and other complementary information available until 2010. It aims to provide the FAO Committee on Fisheries and, more generally, policy-makers, civil society, fishers and managers of world fishery resources with a comprehensive, objective and global review of the state of the living marine resources. ISBN 978-92-5-107023-9 ISSN 2070-7010 FAO 9 789251 070239 I2389E/1/12.11 Cover illustration: Emanuela D’Antoni FAO FISHERIES AND Review of the state AQUACULTURE TECHNICAL of world marine PAPER fishery resources 569 Marine and Inland Fisheries Service Fisheries and Aquaculture Resources Use and Conservation Division FAO Fisheries and Aquaculture Department FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

EIA for Offshore Seismic Survey
MINISTRY OF ENVIRONMENT AND TOURISM (MET) ENVIRONMENTAL CLEARANCE CERTIFICATE (ECC) APPLICATION REF No.: APP-00831 CGG Services UK Limited Final Environmental Impact Assessment (EIA) Vol. 2 of 3 Report to Support the Application for Environmental Clearance Certificate (ECC) for the Proposed Multiclient 2D and 3D Seismic Survey Covering the NORTHERN OFFSHORE NAMIBIA CGG Services UK Limited Crompton Way, Manor Royal Estate, Crawley, West Sussex, RH10 9QN, UNITED KINGDOM CGG Multiclient 2D & 3D Seismic Survey i EIA Report Northern Offshore Namibia -Nov 2019 Summary Information Ministry of Environment and Tourism (MET) Environmental Clearance Certificate (ECC) Application Ref No.: APP-00831 Permit Applied For Environmental Clearance Certificate (ECC) Proponent CGG Services UK Limited Regulatory Framework Environmental Management Act, (EMA), 2007, (Act No. 7 of 2007) and Environmental Impact Assessment (EIA) Regulations No. 30 of 2012 Type of Listed Activities 2D and 3D Multiclient Seismic Surveys for Petroleum Exploration Proposed Project Location Offshore, Northern Namibia, West Coast of Africa Proponent Address CGG Services UK Limited Crompton Way, Manor Royal Estate, Crawley, West Sussex, RH10 9QN, UNITED KINGDOM Environmental Consultants Risk-Based Solutions (RBS) CC (Consulting Arm of Foresight Group Namibia (FGN) (Pty) Ltd) 41 Feld Street Ausspannplatz Cnr of Lazarett and Feld Street P. O. Box 1839, WINDHOEK, NAMIBIA Tel: +264 - 61- 306058; FaxMail: +264-886561821 Mobile: +264-811413229 /812772546 Email: [email protected] Global Office / URL: www.rbs.com.na Environmental Assessment Practitioner (EAP) Dr. Sindila Mwiya PhD, PG Cert, MPhil, BEng (Hons), Pr Eng REPORT CITATION: Risk-Based Solutions (RBS), 2019. Environmental Impact Assessment (EIA) Report to Support the Application for Environmental Clearance Certificate (ECC) for the for the Proposed Multiclient 2D and 3D seismic survey operations Covering the Northern Offshore Namibia. -

A Molecular Analysis of Atlantic Menhaden (Brevoortia Tyrannus) Stock Structure
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2008 A Molecular Analysis of Atlantic Menhaden (Brevoortia tyrannus) Stock Structure Abigail J. Lynch College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Molecular Biology Commons, and the Oceanography Commons Recommended Citation Lynch, Abigail J., "A Molecular Analysis of Atlantic Menhaden (Brevoortia tyrannus) Stock Structure" (2008). Dissertations, Theses, and Masters Projects. Paper 1539617866. https://dx.doi.org/doi:10.25773/v5-rw45-j035 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. A Molecular Analysis of Atlantic Menhaden (Brevoortia tyrannus) Stock Structure A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Abigail J. Lynch 2008 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science i igail J. Lynch Approved by the Committee, July 21, 2008 John Ei Graves, Ph.D. Committee Chairman/Advisor cD owell, Ph.IJNJan R. cDowell, Ph.IJNJan Robert -

Thesis Sci 2021 De Vos Lauren.Pdf
BIODIVERSITY PATTERNS IN FALSE BAY: AN ASSESSMENT USING UNDERWATER CAMERAS Lauren De Vos Thesis Presented for the Degree of DOCTOR OF PHILOSOPHY Universityin the Department ofof Biological Cape Sciences Town UNIVERSITY OF CAPE TOWN October 2020 Supervised by Associate Professor Colin Attwood, Dr Anthony Bernard and Dr Albrecht Götz The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town ii DECLARATIONS PLAGIARISM DECLARATION I know the meaning of plagiarism and declare that all the work in this thesis, save for that which is properly acknowledged, is my own. This thesis has not been submitted in whole or in part for a degree at any other university. Signature: Date: 31 October 2020 iii RESEARCH DECLARATION Research was conducted inside the Table Mountain National Park (TMNP) marine protected area (MPA) with permission from South African National Parks (SANParks). Permit Number: CRC-2014-012. Portions of the fish baited remote underwater mono-video system (mono-BRUVs) data, those pertaining to chondrichthyans, used in this thesis are published in De Vos, L., Watson, R.G.A., Götz, A. & Attwood, C.G. 2015. Baited remote underwater video system (BRUVs) survey of chondrichthyan diversity in False Bay, South Africa. African Journal of Marine Science. 37(2): 209-218. -

3D Model Set by Ken Gilliland Introduction Nature’S Wonders Fish Introduces Three of the Most Common “Oily” Fish Species; Anchovy, Herring and Sardine
3D model set by Ken Gilliland Introduction Nature’s Wonders Fish introduces three of the most common “oily” fish species; anchovy, herring and sardine. These three fish has provided important food sources to seabirds, other fish and humans for thousands of years. The set includes the three fully rigged fish, plus a canned fish tin (which peels back to open) and the packed fish contents. Both Poser and DAZ Studio versions are included and Iray, Superfly, 3Delight and Firefly renderers are supported. Overview and Use This set uses a common models to recreate digitally the fish species included in this volume. Each species uses specific morphs from the generic model to single-out its unique features. Models included in this volume: o Natures Wonders Anchovy Base o Natures Wonders Herring Base o Natures Wonders Sardine Base o Fish Tin o Fish Tin Contents Using Fish in the Set All fish were created in Character format. 1. Go to the Nature’s Wonders Fish folder. 2. Select the “Fish of the World” folder and then appropriate renderer folder. 3. Click and load the fish. 4. Go back to the “Fish of the World” folder and select the “Poses” folder. 5. Apply a pose to the selected fish. Using the Fish Tin and Tin Contents The Fish Tin model is fully articulated model, although the majority of the body parts are hidden. Master controls for the hidden parts can be found in the main (BODY) section. The controls primarily lift and /or roll back the lid. The Fish Tin Contents model is a conforming part in Poser (similar to clothing items).