MAKE YOUR OWN ORANGE DRINK an EXPERIMENT in DETERMINING HOW ADDITIVES AFFECT OUR FOOD ©2000, 1999, 1996 by David A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mondelez International Announces $50 Million Investment Opportunity for UK Coffee Site
November 7, 2014 Mondelez International Announces $50 Million Investment Opportunity for UK Coffee Site - Proposal coincides with Banbury coffee plant's 50th anniversary - Planned investment highlights success of Tassimo single-serve beverage system - Part of a multi-year, $1.5 billion investment in European manufacturing BANBURY, England, Nov. 7, 2014 /PRNewswire/ -- Mondelez International, the world's pre-eminent maker of chocolate, biscuits, gum and candy as well as the second largest player in the global coffee market, today announced plans to invest $50 million (£30 million) in its Banbury, UK factory to build two new lines that will manufacture Tassimo beverage capsules. Tassimo is Europe's fastest growing single-serve system, brewing a wide variety of beverages including Jacobs and Costa coffees and Cadbury hot chocolate. The decision is part of Mondelez International's multi-year investment in European manufacturing, under which $1.5 billion has been invested since 2010. The planned investment will create close to 80 roles and coincides with the 50th anniversary of the Banbury factory, which produces coffee brands such as Kenco, Carte Noire and Maxwell House. The Tassimo capsules produced in Banbury will be exported to Western European coffee markets in France and Spain as well as distributed in the UK. "Tassimo is a key driver of growth for our European coffee business, so this $50 million opportunity is a great one for Banbury," said Phil Hodges, Senior Vice President, Integrated Supply Chain, Mondelez Europe. "Over the past 18 months, we've made similar investments in Bournville and Sheffield, underscoring our commitment to UK manufacturing. -

2004 Annual Meeting of Stockholders Kraft Foods Inc. April 27, 2004 East Hanover, New Jersey
2004 Annual Meeting of Stockholders Kraft Foods Inc. April 27, 2004 East Hanover, New Jersey Louis Camilleri, Chairman of the Board: Good morning, ladies and gentlemen. Thank you for coming. I am Louis Camilleri, Chairman of the Board of Kraft Foods Inc. The 2004 Annual Meeting of Stockholders is now called to order. It is my pleasure to welcome the stockholders here in East Hanover, as well as those of you who are joining us via live webcast. I’d like to introduce the executives here on the stage with me. First David Johnson, President of Kraft’s North America Commercial unit. Next is Hugh Roberts, President of our International Commercial unit. Also with us is Marc Firestone, Kraft’s Executive Vice President, General Counsel and Corporate Secretary. As you know, Roger Deromedi, Kraft’s CEO, has been out on leave, with a serious viral infection. We are delighted that he is making a complete recovery and will be back in the office May 10 to resume his full responsibilities. Next, I would like to introduce Larry Borek of PricewaterhouseCoopers, our auditors. He is in the audience, and he will be available to answer questions after the meeting. Larry, will you please stand? Thank you. The agenda and procedures for the meeting have been placed on your seat. I particularly want to remind everyone of the time limits for questions and comments and the fact that all questions and comments should be addressed to me as Chairman. The secretary will now present certain formal documents. Marc… Marc Firestone, Executive Vice President, General Counsel and Corporate Secretary: Thank you, Mr. -

Enel Green Power's Renewable Energy Is Part of the History of Mondelēz International's Business Unit in Mexico
Media Relations T (55) 6200 3787 [email protected] enelgreenpower.com ENEL GREEN POWER'S RENEWABLE ENERGY IS PART OF THE HISTORY OF MONDELĒZ INTERNATIONAL'S BUSINESS UNIT IN MEXICO • Enel Green Power supplies up to 77 GWh annually to two Mondelēz International factories with wind energy from its 200 MW Amistad I wind farm located in Ciudad Acuña, Coahuila. • Thanks to this relationship, Mondelēz International has avoided the emission of approximately 33,000 tons of CO2 per year. Mexico City, October 7th, 2020 – Enel Green Power México (EGPM), the renewables subsidiary of Enel Group, joins the celebration of the 8th anniversary of Mondelēz International in the country, by commemorating two years of successful collaboration through an electric power supply contract. Derived from this contract, Mondelēz International has received up to 77 GWh per year of renewable energy to its factories located in the State of Mexico and Puebla. Thanks to the renewable energy supplied by EGPM´s Amistad I wind farm; Mondelēz International has avoided the emission of around 33,000 tons of CO2 per year, equivalent to almost 80% of its emission reduction target for Latin America in 2020. Similarly, this energy is capable of producing approximately more than 100,000 tons annually of product from brands such as Halls, Trident, Bubbaloo, Oreo, Tang and Philadelphia and is enough to light approximately 33,000 Mexican homes for an entire year. “It is an honor for Enel Green Power México to contribute to Mondelēz International environmental objectives and efforts to accelerate energy transition in the country. Today more and more companies are convinced that renewable energies are not only sustainable, but also profitable, which is why this type of agreements serve as a relevant growth path for clean sources in Mexico”, stated Paolo Romanacci, Country Manager of Enel Green Power Mexico. -

ORDER GUIDE CATALOG General Index TOBACCO DEPARTMENT 1
ORDER GUIDE CATALOG General Index TOBACCO DEPARTMENT 1 CANDY DEPARTMENT 13 HEALTH & BEAUTY AIDS 26 GENERAL MERCHANDISE 36 GROCERY DEPARTMENT 43 STORE SUPPLIES 59 GENERAL INDEX TOBACCO DEPARTMENT 1 MINTS - LIFESAVERS 15 MUSCLE RUBS - SPORTS CREAMS 27 CIGAR - 2/PK SPECIAL 12 MINTS - TIC TAC 15 NASAL SPRAY 28 CIGARETTE PAPERS / TUBES 1 MISC. COOKIES 22 PAIN RELIEF - MENSTRUAL 26 CIGARS, BOX 6 NABISCO BIG BAGS 99c 24 PETROLEUM JELLY 29 CIGARS, FILTERED 3 NABISCO GROCERY PACK 24 PREGNANCY TESTS 33 CIGARS, PACKS 4 NABISCO SINGLE SERVE 24 RAZOR BALDES 30 DRY SNUF, POCKET & LARGE CAN 8 NABISCO SLUG PACKS 24 RAZORS 30 LIGHTERS 12 NOVELTY CANDY 17 SANITARY NAPKINS 32 R.Y.O. ACCESSORIES 2 PEG BAG CANDY 20 SHAMPOO 31 SMOKER ITEMS, MISC 2 PICKLES 25 SHAVING CREAM 30 SPECIALTY CIGARETTES 3 SATHERS CANDY 2/1.00 21 SINUS MEDICINE 26 TOBACCO LARGE TINS 11 SEEDS & NUTS 23 SKIN CARE / FACE-HAND CREAM 31 TOBACCO RYO CIGARETTE BLEND 10 SNACKS 23 SLEEPING AIDS 29 TOBACCO, CHEWING 8 THEATRE PACK CANDY 20 STIMULANTS 28 TOBACCO, PIPE BAG & POUCH 9 SUNTAN LOTION 31 TOBACCO, PLUG 9 HEALTH & BEAUTY AIDS 26 SUPPOSITORIES / OINTMENTS 28 TOBACCO, ROLL SNUFF 7 AFTER SHAVE 31 THROAT SPRAY & LOZENS 28 ALLERGY MEDICATION 27 TOOTHACHE REMEDY 35 CANDY DEPARTMENT 13 ANALGESIC 50/2 BOX 26 TOOTHBRUSHES 35 19 ANALGESICS - CHILDREN 26 TOOTHPASTE 34 5 SUGARFREE GUM 13 ANALGESICS - STANDARD 26 ANTACIDS - TUMS 15 ANTACIDS 27 GENERAL MERCHANDISE 36 ANTACIDS- ROLAIDS 15 ANTI-DIHARRHEAL MEDICINE 27 AIR FRESHENERS 39 CANDY - 25c 16 ANTISEPTICS 29 AUTO ACCESSORIES 39 CANDY - 5c 16 BABY -

Chocolatiers and Chocolate Experiences in Flanders & Brussels
Inspiration guide for trade Chocolatiers and Chocolate Experiences IN FLANDERS & BRUSSELS 1 We are not a country of chocolate. We are a country of chocolatiers. And chocolate experiences. INTRODUCTION Belgian chocolatiers are famous and appreciated the world over for their excellent craftmanship and sense of innovation. What makes Belgian chocolatiers so special? Where can visitors buy a box of genuine pralines to delight their friends and family when they go back home? Where can chocolate lovers go for a chocolate experience like a workshop, a tasting or pairing? Every day, people ask VISITFLANDERS in Belgium and abroad these questions and many more. To answer the most frequently asked questions, we have produced this brochure. It covers all the main aspects of chocolate and chocolate experiences in Flanders and Brussels. 2 Discover Flanders ................................................. 4 Chocolatiers and shops .........................................7 Chocolate museums ........................................... 33 Chocolate experiences: > Chocolate demonstrations (with tastings) .. 39 > Chocolate workshops ................................... 43 > Chocolate tastings ........................................ 49 > Chocolate pairings ........................................ 53 Chocolate events ................................................ 56 Tearooms, cafés and bars .................................. 59 Guided chocolate walks ..................................... 65 Incoming operators and DMC‘s at your disposal .................................74 -

J&J Snack Foods Introduces OREO® Churros to Be Sold to The
Contact: Alissa Davis J&J Snack Foods Corp 856-532-6615 [email protected] FOR IMMEDIATE RELEASE J&J Snack Foods Introduces OREO® Churros To be sold to the Foodservice Industry, Nationwide! Pennsauken, NJ – (November 4, 2014) J&J Snack Foods Corp. (NASDAQ: JJSF) today proudly announced a partnership with Mondelez International to introduce OREO® Churros. With a crispy exterior, warm soft interior and real OREO® cookie pieces in every bite, new OREO® churros have a “just-baked” OREO® cookie taste. This innovative new snack will be sold nationwide in the foodservice channel. The new churros made their debut at the National Association of Convenience Stores (NACS) Show in Las Vegas earlier this month. Available in traditional churro sticks, double-twisted churros and bite-size churros, these delicious treats are ideal for quick service restaurants, convenience stores, sports & leisure venues and all segments within the foodservice industry. The churros can be served with OREO® cookie creme dip, supplied by J&J Snack Foods, rolled in sugar or topped with a favorite ice cream. “As the leading churro manufacturer, we are excited about our new relationship with the world famous OREO® cookie brand,” said Jerry Law, J&J Snack Foods Senior Vice President. “OREO® cookie and churro fans alike will not be disappointed in the latest snacking mash up.”. 102 years old, , OREO® is the #1 brand on dessert menus1 and #1 selling cookie.2 J&J Snack Foods is the leading manufacturer of churros, often referred to as a Spanish donut, under Tio Pepe’s Churros and California Churros brands. -
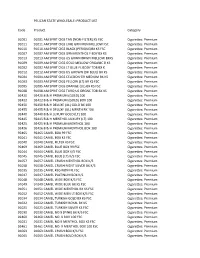
(NON-FILTER) KS FSC Cigarettes: Premiu
PELICAN STATE WHOLESALE: PRODUCT LIST Code Product Category 91001 91001 AM SPRIT CIGS TAN (NON‐FILTER) KS FSC Cigarettes: Premium 91011 91011 AM SPRIT CIGS LIME GRN MEN MELLOW FSC Cigarettes: Premium 91010 91010 AM SPRIT CIGS BLACK (PERIQUE)BX KS FSC Cigarettes: Premium 91007 91007 AM SPRIT CIGS GRN MENTHOL F BDY BX KS Cigarettes: Premium 91013 91013 AM SPRIT CIGS US GRWN BRWN MELLOW BXKS Cigarettes: Premium 91009 91009 AM SPRIT CIGS GOLD MELLOW ORGANIC B KS Cigarettes: Premium 91002 91002 AM SPRIT CIGS LT BLUE FL BODY TOB BX K Cigarettes: Premium 91012 91012 AM SPRIT CIGS US GROWN (DK BLUE) BX KS Cigarettes: Premium 91004 91004 AM SPRIT CIGS CELEDON GR MEDIUM BX KS Cigarettes: Premium 91003 91003 AM SPRIT CIGS YELLOW (LT) BX KS FSC Cigarettes: Premium 91005 91005 AM SPRIT CIGS ORANGE (UL) BX KS FSC Cigarettes: Premium 91008 91008 AM SPRIT CIGS TURQ US ORGNC TOB BX KS Cigarettes: Premium 92420 92420 B & H PREMIUM (GOLD) 100 Cigarettes: Premium 92422 92422 B & H PREMIUM (GOLD) BOX 100 Cigarettes: Premium 92450 92450 B & H DELUXE (UL) GOLD BX 100 Cigarettes: Premium 92455 92455 B & H DELUXE (UL) MENTH BX 100 Cigarettes: Premium 92440 92440 B & H LUXURY GOLD (LT) 100 Cigarettes: Premium 92445 92445 B & H MENTHOL LUXURY (LT) 100 Cigarettes: Premium 92425 92425 B & H PREMIUM MENTHOL 100 Cigarettes: Premium 92426 92426 B & H PREMIUM MENTHOL BOX 100 Cigarettes: Premium 92465 92465 CAMEL BOX 99 FSC Cigarettes: Premium 91041 91041 CAMEL BOX KS FSC Cigarettes: Premium 91040 91040 CAMEL FILTER KS FSC Cigarettes: Premium 92469 92469 CAMEL BLUE BOX -

The History of Kraft Foods Inc
The History of Kraft Foods Inc. All About Kraft Learn everything there is to know about Kraft: like who we are, how you can reach us and what we’re doing in your community. Kraft Foods Inc. is a company with many different roots and founders, all sharing a commitment to quality, a willingness to take risks and a spirit of innovation. Among the products now sold by Kraft Foods Inc. are so many “firsts” and innovations that a history of the company is almost a history of the food industry. Kraft traces its history to three of the most successful food entrepreneurs of the late 19th and early 20th centuries — J.L. Kraft, who started his cheese business in 1903; C.W. Post, who founded Postum Cereal Company (later renamed General Foods Corporation) in 1895; and Oscar Mayer, who began his meat business in 1883. The Story of J.L. Kraft The history of KRAFT goes back to 1903, when, with $65 in capital, a rented wagon and a horse named Paddy, J.L. Kraft started purchasing cheese at Chicago’s Water Street wholesale market and reselling it to local merchants. Within a short time, four of J.L. Kraft’s brothers joined him in the business, and, in 1909, they incorporated as J.L. Kraft & Bros. Co. In 1914, J.L. Kraft and his brothers purchased their first cheese factory in Stockton, Illinois. In 1915, they began producing processed cheese in 3-1/2 and 7-3/4 ounce tins. J.L. Kraft’s method of producing processed cheese was so revolutionary, in 1916 he obtained a patent for it and in 1917 the company started supplying cheese in tins to the U.S. -

Mondelez International Joins Chocolate Industry's 'Cocoaaction' Through World Cocoa Foundation
May 23, 2014 Mondelez International Joins Chocolate Industry's 'CocoaAction' Through World Cocoa Foundation - Coordinates Support from 12 Largest Global Cocoa Companies - Focuses on Boosting Supply Chain Productivity and Community Development - Complements Mondelez International's $400 Million Cocoa Life Sustainability Initiative DEERFIELD, Ill., May 23, 2014 /PRNewswire/ -- Mondelez International, the world's largest chocolate company and a proud member of the World Cocoa Foundation since 2004, is lending its support for the chocolate industry's "CocoaAction" sustainability strategy announced earlier this week during the visit by its board of directors in West Africa. CocoaAction brings together 12 of the largest companies in the cocoa industry under the World Cocoa Foundation to voluntarily coordinate and align their sustainability efforts, boost their impact and contribute to building a rejuvenated and economically viable cocoa sector. "As a founding member of the World Cocoa Foundation, we're proud to have played an active role in shaping CocoaAction," said Bharat Puri, President, Global Chocolate, Gum and Candy. "We believe in the power of major chocolate and cocoa companies working together to maximize the industry's ability to create thriving cocoa communities and help secure the future of the cocoa industry. We're pleased our signature Cocoa Life program, launched in 2012, aligns with the CocoaAction strategy, and we welcome the support from the governments of Ghana and Cote d'Ivoire for CocoaAction." Mondelez International will continue to expand its Cocoa Life sustainability program, a $400 million, 10-year effort plan based on its successful Cadbury Cocoa Partnership in Ghana, which has promoted gender equality in cocoa production since 2008. -

This Festive Season, Oreo Launches a New TVC for the Brand New Limited Edition Flavor ‘Oreo Roast Almond Crème’
Media Release: Nov 2015/Mum This festive season, Oreo launches a new TVC for the brand new limited edition flavor ‘Oreo Roast Almond Crème’ Mondelez India Foods Pvt. Ltd (Formerly Cadbury India Ltd) launched an endearing new television commercial this festive season for its recently launched limited edition flavor, Oreo Roast Almond Crème. Conceptualized by Interface Communications, the TVC is a light hearted story of siblings going lantern shopping. While the sister is interested in choosing the right lantern, the brother just wants to get back home……when he ‘discovers’ the new Oreo Roast Almond Crème. Chella Pandyan, Associate Director, Marketing, Biscuits, India and Kids Fuel, AP shared, “This festive season, we are delighted to present to our consumers a special offering to make the season even more special for them… the new Oreo Roast Almond Crème limited edition. The new TVC is built on a simple insight around togetherness amongst siblings while unveiling the new limited edition flavor. It is a warm and lighthearted TVC that I believe everyone would connect with.” Commenting on this new TVC, Joe Thaliath, COO, Interface Communications, said, “Across the world, innovative limited edition Oreo flavors are sought after, and with festivities around the corner, we took the opportunity to bring this concept to India. The new limited edition Oreo Roast Almond Crème makes for the perfect treat and drives the brand further.” Robby Mathew, Chief Creative Officer of Interface Communications, added, “Oreo is all about family and the bonds that -

Kraft Reports Strong Revenue Growth in 2007; Enters 2008 with Good Momentum
Contacts: Lisa Gibbons (Media) Christopher M. Jakubik (Investors) 847-646-4538 847-646-5494 Kraft Reports Strong Revenue Growth in 2007; Enters 2008 with Good Momentum • 2007 net revenues up 8.4%; organic net revenues1 grew 5.1%, above guidance. • 2007 diluted EPS $1.62, down 12.4%; $1.82 excluding items,1 in line with guidance. • Fourth-quarter net revenues increased 10.9%; organic net revenues grew 6.2%. • Fourth-quarter diluted EPS $0.38; $0.44 excluding items. • 2008 guidance of at least 4% organic revenue growth and at least $1.56 diluted EPS, or $1.90 excluding $0.34 cents of restructuring costs. NORTHFIELD, Ill. – January 30, 2008 – Kraft Foods Inc. (NYSE: KFT) today reported fourth-quarter and full-year 2007 results that reflect accelerated revenue growth in the first year of its three-year transformation plan. Volume growth improved as the year progressed due to the company’s investments in quality, innovation and brand building. However, volume and pricing gains were not able to fully offset significantly higher input costs, primarily dairy, and the company's investments, resulting in earnings declines for fourth quarter and full year. “We are off to an excellent start in our efforts to return Kraft to reliable growth,” said Irene Rosenfeld, Chairman and Chief Executive Officer. “We’ve shown that our investments in product quality, marketing and innovation lead to accelerated volume growth, better product mix and improved market share trends. At the same time, we’ve significantly reduced our cost structure and strengthened our portfolio with the acquisition of Danone’s global biscuit business and the announcement to exit the Post cereal business. -

Lonely Planet's Global Chocolate Tour 1 Preview
CONTENTS Introduction 3 Costa Rica 60 Europe 146 The Beans 4 Cuba 64 Austria 148 Cacao to Chocolate 6 Ecuador 66 Belgium 152 INTRODUCTION Types of Chocolate 8 Honduras 70 Eastern Europe 160 From camel milk chocolate in Dubai to honeycomb We couldn't cover every worthy Swiss chocolatier or Glossary 11 Mexico 72 France 164 chocolate in Australia, single-origin chocolate ice incredible Parisian chocolate boutique, but we included Nicaragua 80 Germany 176 cream in San Francisco and chocolate-covered blueberries favourites from Lonely Planet writers across the world. The Africa & The Middle East 12 Peru 82 Iceland 184 from Trappist Monks in Quebec, the world of chocolate has major cacao-growing countries are represented as well, Cote d’Ivoire 14 Chocomuseos 84 Ireland 186 never been more diverse...or more delectable. Innovative often with tours of cacao farms where it's possible to see Ghana 16 USA 86 Italy 188 chocolatiers are thinking up novel ingredient combinations the crop as it's grown and harvested. While most production Israel & Palestinian Territories 18 Top Chocolate Festivals 116 Netherlands 194 from Ho Chi Minh City to Texas and finding new means of of chocolate is done elsewhere and growers in places like São Tomé & Príncipe 22 Portugal 200 sourcing from and supporting small cacao farmers in the Côte d'Ivoire and Costa Rica primarily export the raw crop South Africa 24 Asia 118 Spain 202 race to elevate each bite into chocolate heaven. Yet not without much in-country chocolate production of their own, United Arab Emirates 30 India 120 Switzerland 206 every chocolate destination in this book is a craft bean-to- new bar-makers are popping up all over to challenge the Top Hot Chocolates 32 Indonesia 122 United Kingdom 212 bar maker; beloved Hershey's Chocolate World, chocolate- traditional paradigm and capture more of the revenue from Japan 124 Top Flavour Pairings 228 themed hotels and classic old-world cafes serving famous the chocolate trade domestically.