Amino Acid, Mineral, and Polyphenolic Profiles of Black Vinegar, and Its
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Specification
PRODUCT SPECIFICATION Page 1 of 2 Prepared for TENDENCIA GASTRONOMICA Product #: 70600 - ROLAND PREMIUM MODENA BALSAMIC VNGR Generated: December 30, 2010 Updated August 10, 2010 Manufacturer ID: 41224 AMERICAN ROLAND FOOD CORP. WOODCLIFF LAKE NJ 07675 Phone: +1 (212) 741-8295 Fax: +1 (212) 206-0589 PRODUCT INFORMATION Product Category: 00050-11610 UPC - Shipping: 10041224706009 UPC - Unit: 041224706002 Unit Pack: 2 / 5 L Carton Kosher: Gross Weight: 27 lbs. Drained Weight: N/A Net Volume: 10 L Case Dimensions: H: 11" W: 7.5" L: 12" Cubic Feet: 0.573 Unit Dimensions: H: 10" W: 6" L: 6.5" Gross Unit Weight: 0 oz. Unit Color: Other: Pallet: Tie 18 High 4 Extra 0 Pallet Quantity: 72 Shelf Life Unopened: 36 MONTHS Storage Temp (F): 70 Opened: 12 MONTHS NUTRITIONAL INFORMATION Serving Size: 1.00 TBSP (15mL) DR Servings per Case: 0 per Unit: 0 %Daily %Daily Value* Value* Calories 20kcal Sugar 4g Calories from Fat 0kcal Protein 0g Total Fat 0g 0% Vitamin A 0% Saturated Fat 0g 0% Vitamin C 0% Trans Fat 0g Calcium 0% Cholesterol 0mg 0% Iron 0% Sodium 0mg 0% Moisture N/A Total Carbohydrates 5g 2% Ash N/A Dietary Fiber 0g 0% *Percent Daily Values are based on a 2,000-calorie diet. Your daily values may be higher or lower depending on your calorie needs. All information contained herein is the property of American Roland Food Corp. and may not be copied without permission. Above information is based on a compilation of data and is formatted in compliance with FDA. PRODUCT SPECIFICATION Page 2 of 2 Prepared for TENDENCIA GASTRONOMICA Product #: 70600 - ROLAND PREMIUM MODENA BALSAMIC VNGR Generated: December 30, 2010 Updated August 10, 2010 Manufacturer ID: 41224 AMERICAN ROLAND FOOD CORP. -

New OVPRE Officials Designated the Designation of a New Set of Officials for the Year 2019 Meanwhile, Dr
ISSN 2651-8023 The official monthly newsletter of the Office of the Vice President for Research and Extension VOL. 2 No. 1 January 2019 New OVPRE officials designated The designation of a new set of officials for the year 2019 Meanwhile, Dr. Yolanda A. Ilagan, former director of the was recently released by the Office of the President thru an Knowledge Management Center (KMC), has been designated as office memorandum. Most of the officials retained their posts the new Director for Research replacing Dr. Ma. Cynthia R. dela while others were either transferred to another office/campus or Cruz who is now the campus administrator of CvSU Imus. On replaced. the other hand, Dr. Ma. Fatima I. Cruzada replaced Dr. Ilagan as the new Director of KMC. She is a faculty researcher and Research and Extension as a major unit of the University welcomes the newly appointed directors. Dr. Ruel M. Mojica instructor at the Department of Biological SciencesCollege of retained his post as Vice President for Research and Extension Arts and Sciences. and Dr. Lilibeth P. Novicio as Director for Extension. Engr. Gerry M. Castillo, former staff of the Office of the Vice President for Research and Extension (OVPRE) and instructor at the College of Engineering and Information GMA News TV features kaong Technology (CEIT), was assigned as director of the National GMA News TV, thru its program Pinas Sarap aired on Coffee Research, Development and Extension Center January 17, 2019 and hosted by Ms. Kara David, featured kaong (NCRDEC) whose previous director was Dr. Miriam D. Baltazar. vinegar and the projects and activities of the CvSU Sugar Palm The new assignments will take effect on February 1, 2019. -

Antioxidant Activities of Different Types of Vinegars
Antioxidant Activities of Different Types of Vinegars OBJECTIVE: To study the free radical scavenging activity and ferric reducing power of aqueous extracts of different types of Farjana Yasmin1*, Khairul Niza Abdul Razak2, Nor Adlin Yusoff1 vinegar at different concentrations. 1 Advanced Medical and Dental Institute (AMDI), Universiti Sains RESULTS AND DISCUSSION Malaysia, Penang, Malaysia.2 School of Pharmaceutical Sciences, DPPH Universiti Sains Malaysia, Penang, Malaysia. 150 Apple cider *Corresponding author: [email protected] balsamic 100 brown rice INTRODUCTION: Vinegars are functional foods that are distilled white malt 50 widely consumed. Preclinical animal studies have also reported nipah the effects of different vinegars on metabolic parameters (Yusoff red wine 0 et al., 2015). In addition, high blood sugar was also reported to 0.0 0.5 1.0 1.5 2.0 2.5 mg/ml cause increased free radicals and reduced antioxidant markers. Figure 1 showed that Balsamic Vinegar is the strongest effect Polyphenols and melanoidins, respectively, also have the among of the total DPPH activity of different vinegars. The antioxidant properties of vinegars derived from raw materials different types of vinegar showed a trend of free radical and fermentation processes (Chen et al. 2016). So, this study scavenging activity as follows: BV > NPV > RV > MV > ACV > was designed to evaluate the antioxidant effects of different BRV > DWV. types of vinegars that may help to control postprandial glucose 90 level. 80 MATERIALS AND METHODS 70 Vinegar samples and sample preparation 60 Seven vinegars were obtained from local retailers as follows: 50 Apple cider vinegar (ACV), balsamic vinegar (BV), brown rice 40 30 vinegar (BR), distilled white vinegar (DW), malt vinegar (MV), Concentration mg/ml 20 nipa palm vinegar (NPV) and red wine vinegar (RWV). -

Homemade Vinaigrettes & Dressings
Homemade Vinaigrettes & Dressings DRESS IT UP! 9 Homemade Vinaigrettes & Dressings Vinaigrettes and dressings (we use the terms interchangeably) are a wonderful and simple way to add flavor and nourishment to the meals you make. Beyond their traditional role in salads, ratio is perfect and packs a nice vinegar you can use them to marinade proteins, punch. You can also thin out the vinaigrette as a flavor component for stir fries, a with water if you wish. light enhancement to steamed or roasted vegetables and even as a light dipping Ingredient Choice sauce for raw vegetables. There are many different oils to use from and substitutions can be made in every The beauty of making your own dressing recipe. Extra-virgin olive oil is a top choice is having the choice to use the freshest for homemade dressing, but you can also ingredients and mixing them up in choose avocado oil as a change. Walnut or interesting ways. Dressings also provide hazelnut oil and beautiful pungency to salad an easy way to add health supporting dressings as does toasted sesame oil which (and gene talking) herbs and spices, giving adds those delightful nuances to Asian your body more good food “information” inspired dishes. to work with. Once you make your own dressings, it’s hard to think of reaching for Vinegars vary in acidity and flavor, and store-bought versions which frequently there are many fantastic choices like contain additives and stabilizers. champagne, balsamic, red wine, sherry, apple cider, rice wine and white vinegar. Citrus such A classic vinaigrette is a basic type of as fresh lemons, oranges or limes, can be a dressing that combines oil and vinegar, great addition or substitution for a vinegar. -

Cheese Making Sources: One-Hour Cheese by Claudia Lucero; Edgewood Creamery Website; Science.Howstuffworks.Com;
The Basic Science (It’s Magic!) Behind Cheese Making Sources: One-Hour Cheese by Claudia Lucero; Edgewood Creamery website; Science.HowStuffWorks.Com; Instructional video available on Driftwood Public Library’s new YouTube Channel: https://www.youtube.com/watch?v=OY39X-eSLLI&t=144s “Cheesemaking is the controlled process of removing water from milk. This process concentrates the milk’s protein, fat and other nutrients and increases its shelf life. Cheesemaking is one of the earliest examples of biotechnology.” – Edgewood Creamery Like sourdough bread, making cheese is one of the earliest forms of microbiology. The transformation of milk into cheese is one of the most extraordinary of all human discoveries. No one knows exactly when, but it is believed to have happened at about the same time as the domestication of animals such as goats in the fertile crescent region of the Middle East, around 6,000-7,000BC. It is unknown when the first cheese was made, but the legend goes that a nomad was carrying milk in a pouch made of a calf’s stomach lining. Over time (and at the right temperature) the milk fermented. Not wanting to waste the chunky milk, the nomad tried it and liked it. This legend about the first cheese is not unlike that of the first sourdough leavened bread. The components of Cheese: Milk: Made up of water, fats, proteins, bacteria, lactose (a sugar), minerals and more. Unpasteurized milk also contains the enzyme lactase, which helps you digest milk. Salt: Besides adding flavor, “salt is also a great preservative and, under the right conditions, can help turn a previously highly perishable substance (milk) into a stable cheese that doesn’t require regular refrigeration.” Note: When making one-hour cheese, we only use salt for flavor and to control the amount of whey we leave in the curds for texture. -

Continued Consumption of Beverage Containing Black Vinegar Reduces Fatigue After Exercise Confirmed in a Human Clinical Study
NEWS RELEASE September 6, 2018 Continued Consumption of Beverage Containing Black Vinegar Reduces Fatigue after Exercise Confirmed in a human clinical study ITO EN, LTD. (president: Daisuke Honjo; head office: Shibuya-ku, Tokyo) has confirmed that the continued consumption of black-vinegar-based beverages alleviates fatigue after exercise in a human clinical study. Details of the study results will be presented at the 73rd Annual Meeting of the Japanese Society of Physical Fitness and Sports Medicine in Fukui City on Friday, September 7. Scientific verification of black vinegar’s effect of reducing fatigue Vinegar, including black vinegar, is a traditional fermented food that is used as seasoning in a wide variety of food. Recently, consumers have become more interested in the health effects of vinegar, enjoying diluted vinegar as a beverage to lessen fatigue in daily life or after exercise. However, it has not been sufficiently scientifically verified whether vinegar can decrease tiredness. ITO EN performed a clinical study using a beverage containing black vinegar to scientifically verify whether the acetic acid in the test beverage (beverage containing black vinegar) effectively alleviated fatigue after exercise. Confirmed reduction of fatigue after exercise following seven continuous days of consumption of a beverage containing black vinegar Results of the clinical study showed that seven continuous days of consumption of the test beverage significantly reduced fatigue for 30 minutes after exercise and before bedtime compared to the control* beverage (see the figure below). Interestingly, stiff shoulders, one of the endpoints, also decreased before sleep and after waking up on the following day, suggesting a connection to the test beverage. -

Download a Booklet of Balsamic Vinegar of Modena Recipes
THE CULINARY INSTITUTE OF AMERICA Recipes from Consorzio Tutela Aceto Balsamico di Modena Recipes developed by The Culinary Institute of America as an industry service to Consorzio Tutela Aceto Balsamico di Modena. TABLE OF CONTENTS RECIPES ...................................................................................................................................................................... 1 BALSAMIC VINAIGRETTE .......................................................................................................................................... 2 BALSAMIC VINEGAR-ROASTED BRUSSELS SPROUTS, PEARL ONIONS AND PINK PEPPERCORNS ........................... 3 FARRO SALAD WITH BALSAMIC APRICOTS, FETA AND PISTACHIOS ...................................................................... 4 TANGY & SPICY ASIAN BALSAMIC MARINADE ...................................................................................................... 5 BALSAMIC BOURBON BBQ SAUCE ........................................................................................................................... 6 DRY RUB FOR BBQ RIBS ........................................................................................................................................... 8 BALSAMIC, SOY AND PLUM MARINADE FOR STEAK ............................................................................................... 9 POUTINE WITH BALSAMIC-SHORTRIB GRAVY & CHEESE CURDS ......................................................................... 10 GREMOLATA .......................................................................................................................................................... -
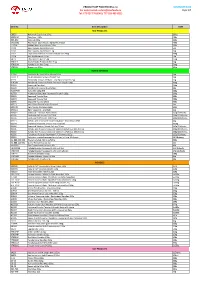
NOVEMBER 2020 Page
PRODUCT LIST TAKO FOODS s.r.o NOVEMBER 2020 For orders email: [email protected] Page 1/7 Tel: 773 027 776 (ENG), 777 926 495 (CZE) Item No. Item Description UoM RICE PRODUCTS 08930 Hakumaki Sushi rice 10 kg 10 kg 303013A Rice flour 400g 400g 839-840 Shiokoji 220g 220g CH16032 Rice Paper 22cm Round Spring-Roll 400g 400g J1371A Malted Rice - Kome Kouji 200g 200g J3100 Rice Toyama Koshihikari 5kg 5kg J3101 Rice Toyama Koshihikari 1 kg 1kg J3102 Toyama Koshihikari Funwari Gohan rice 200g 200g J3104 Rice Akitakomachi 5 kg 5kg J4075 Thai Jasmine Rice 25 kg 25kg YTK019 Koshi Yutaka Premium Rice 5 kg 5kg YTK023G Yutaka Sushi Rice 500g 500g YTK033 Brown rice 10kg 10kg NORI & SEAWEED 17164 Dashi Konbu Dried Kelp (Akaya) 1 kg 1kg CN-11-1 Chuka Wakame Seaweed salad 1kg 1kg K1131 Yamanaka Tokuyo Mehijiki - Dry Hijiki Seaweed 25g 25g K1450B Powdered Seaweed Aonori Premium Grade 100g 100g K1619 Seaweed Ogo Nori 500g K1628 Shredded Seaweed Kizami Nori 50g K1697WR Kaiso Mix 100g WR 100g K1703 Ariake Yaki Bara Nori Seaweed Grade A 100g 100g K2903 Seaweed Tosaka Blue 500g K2904 Seaweed Tosaka Red 500g K2905 Seaweed Tosaka White 500g K3214 Kelp Yama Dashi Konbu (Sokusei) 1kg K3217B Kelp Ma Konbu (Hakodate) 500g K3261A Hijiki Seawood - Me Hijiki 1kg K33021 Seaweed - Yakinori Aya Full Size 125g/50sheets K3305 Sushi nori full sheets 100/230G 230g/100sheets K3306 Sushi nori half sheets 100/125g 125g/100sheets K3313 Kofuku nori Seasoned seaweed Ajitsuke Nori Ohavo 8Pkt 24g K3314 Seasoned seaweed sesame nori chips 8G 30/8g K3340 Seaweed Yakinori Miyabi full -

Olive Oil & Vinegar Infusers
Cook to Inspire Set of 2 Olive Oil & Vinegar Infusers with holder Care Instructions and Recipe Booklet 1 2 #800 Set of 2 Olive Oil & Vinegar Infusers with holder Care Instructions and Recipe Booklet 3 4 INTRODUCTION TO PRODUCT These olive oil and vinegar infusers allow you to customize and create your own flavored oils and vinegar to make a delicious olive oil dip, salad dressing, sauce or anything your heart desires. The process is very simple but requires reading the safety instructions below. There are 2 methods to creating your own olive oil/vinegar–without heat or with heat. Heating your ingredients and oil/vinegar allows you to use the olive oil/vinegar immediately, and leave the herbs in for the flavor to get stronger over time. Making infused olive oil/vinegar without heat is as simple as washing and completely drying your ingredients, bruising the herbs, dropping them into the bottle and covering them with olive oil/vinegar (oil must cover herbs completely). With this method it is better to let your olive oil/vinegar sit for 1-2 weeks before using to get fuller flavor. SAFETY INFO • Read ALL instructions and keep for future reference. • Do not use if glass is broken or cracked in any manner. • Use only for your household, not commercial purposes. • Close supervision is necessary when being used by or near children. • Do not place on or near a hot gas or electric burner or heated oven. CLEANING & CARE • Glass bottle is dishwasher safe, all other pieces are hand wash only. • Make sure entire set dries completely, if there is any water left bacteria can form. -

CPG Sec. 525.825 Vinegar, Definitions - Adulteration with Vinegar Eels
CPG Sec. 525.825 Vinegar, Definitions - Adulteration with Vinegar Eels I. DEFINITIONS BACKGROUND: No standards of identity for vinegar have been established under the Federal Food, Drug, and Cosmetic Act. Historically, definitions have been developed for different types or combinations of types of vinegars. These remain current Agency policy for labeling purposes. One of the landmark court decisions under the Food and Drugs Act of 1906 was that the Supreme Court in the case of U.S. v. 95 Barrels, More of Less, Alleged Apple Cider Vinegar, (265 U.S. 438, 1924), in which the Supreme Court held that vinegar made from dried apples was not the same as that which would have been produced from the apples without dehydration, and that the name "Apple Cider Vinegar" did not represent the article to be what it really was. POLICY: FDA considers the following to be satisfactory guidelines for the labeling of vinegars: Natural vinegars as they come from the generators normally contain in excess of 4 grams of acetic acid per 100 mL. When vinegar is diluted with water, the label must bear a statement such as "diluted with water to _______ percent acid strength", with the blank filled with the actual percent of acetic acid - in no case should it be less than 4 percent. Each of the varieties of vinegar listed below should contain 4 grams of acetic acid per 100 mL.(20oC). VINEGARS: 1. VINEGAR, CIDER VINEGAR, APPLE VINEGAR. The product made by the alcoholic and subsequent acetous fermentations of the juice of apples. 2. WINE VINEGAR, GRAPE VINEGAR. -

Suckling Pig Chinese Plum Puree, Sweet Bean, Chinese 10 Spice 15 Steamed Bao Buns Slow Cooked Pork Belly, Sweet Bean Garlic Glaze 14
To Begin Crisp Lettuce Cups Gulf Prawns, Garlic, Pickled Ginger-Serrano Chili Vinaigrette 16 Maine Lobster Spring Rolls Prawns, Ginger, 10 Spice Honey 16 Crispy Suckling Pig Chinese Plum Puree, Sweet Bean, Chinese 10 Spice 15 Steamed Bao Buns Slow Cooked Pork Belly, Sweet Bean Garlic Glaze 14 Dumplings “Tiny Dumplings” Pork Belly, Black Vinegar, Chili Oil, Ginger, Cilantro Leaves 15 Chili “Dan Dan” Dumplings Organic Chicken, Chili Garlic Sauce, Crushed Peanuts, Green Onion 16 Crystal Chive Dumplings Alaskan King Crab, Shrimp, Kurobuta Pork 18 XO Seafood Dumplings Sweet Shrimp, Alaskan King Crab, Maine Lobster 18 The Sea Broiled Japanese Black Cod Ginseng Honey, Black Sesame Vinaigrette, Chili-Orange Noodle Salad 38 Hong Kong Style Steamed Striped Bass Baby Bok Choy, Lotus Root, Ginger, Aromatic Soy 36 Grilled Santa Barbara Spot Prawns Shallots, Preserved Vegetables, Chili Oil, Soy Black Vinegar 44 Assam Curry Seafood “Hot Pot” Curry Leaves, Cardamom, Yogurt, Fenugreek 46 The Field Crispy Wolfe Ranch Quail “General Tso’s” Chilies, Garlic, Scallion 34 Jidori Chicken Breast Asparagus, Trumpet Mushrooms, Water Chestnuts, XO Garlic, Chilies 28 Prime Filet of Beef Stir Fry Eggplant, Thai Basil, Red Chilies, Crispy Spinach 38 Crispy Skin Kurobuta Pork Belly Wok Fried Green Beans, Golden Chives, Chilies, Garlic 29 Grilled Sonoma Lamb Chops Cilantro Mint Vinaigrette, Ginger, Crispy Garlic, Wild Arugula 52 Szechuan Style Prime Beef Tenderloin Smoked Chili Shallot Sauce, “La You” Hot Oil 49 For The Table Wok Fried Whole Sea Bass Black Pepper Garlic -

Cheese Recipes
cheese recipes RECIPE: MOZZARELLA CHEESE 5. Heat milk slowly to 90°F while stirring. Use thermometer to check temperature. Makes approximately ¾ pound 6. Take pot off burner, add rennet solution INGREDIENTS while stirring slowly top to bottom (fold- ing) for approximately 30 seconds. 1 gallon milk (not ultra-pasteurized) 7. 11/4 cup cool water (chlorine-free) Cover pot with lid and leave undisturbed 11/2 teaspoon citric acid for 5 minutes. 1/4 rennet tablet or 1/4 teaspoon liquid rennet 8. Check the curd. It should look like cus- 1 teaspoon cheese salt (optional) tard, with a clear separation between the (Salt substitutes and/or herbs can be used as curd and the whey. If the curd is too soft an alternative.) or the whey is milky, let sit for a few more minutes. EQUIPMENT 9. Cut the curds in a 1-inch checkerboard pattern with a long knife. 1 gallon stainless steel pot or any non- 10. Place pot back on stove and heat to 105°F aluminum or non-cast iron pot Instant-read thermometer or candy while stirring slowly and gently. Use ther- thermometer mometer to check temperature. Measuring cups and spoons 11. Take the pot off the burner and continue Colander or strainer stirring slowly for 2 – 5 minutes. (More Butter muslin, flour sack towel or cheese cloth time will make a firmer cheese.) 4-quart mixing bowl to collect whey 12. Place the colander over a 4-quart bowl or Slotted spoon pot that will catch the whey. Scoop the Extra spoon curds from your pot with a slotted spoon Long knife and place them in the colander.