Unique Proximal Tubular Cell Injury and the Development of Acute
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

PDI Appendix G Intermediate-Risk Immunocompromised State Ef Ec2
AHRQ QI™ ICD‐10‐CM/PCS Specification Enhanced Version 5.0 1 of 6 Pediatric Quality Indicators Appendices www.qualityindicators.ahrq.gov Pediatric Quality Indicators Appendices APPENDIX G: Intermediate-Risk Immunocompromised State Diagnosis Codes Intermediate-risk immunocompromised state diagnosis codes: (IMMUITD) ICD-9-CM Description ICD-10-CM Description 07022 VIRAL HEPATITIS B W HEPATIC COMA, CHRONIC WO B180 Chronic viral hepatitis B with delta‐agent MENTION OF HEPATITIS DELTA 07023 VIRAL HEPATITIS B W HEPACTIC COMA, CHRONIC W B181 Chronic viral hepatitis B without delta‐agent HEPATITIS DELTA 07044 CHRONIC HEPATITIS C WITH HEPACTIC COMMA B182 Chronic viral hepatitis C 2894 HYPERSPLENISM B520 Plasmodium malariae malaria with nephropathy 28950 DISEASE OF SPLEEN NOS D5702 Hb‐SS disease with splenic sequestration 28951 CHRONIC DIGESTIVE SPLENOMEGALY D57212 Sickle‐cell/Hb‐C disease with splenic sequestration 28952 SPLENIC SEQUESTRATION D57412 Sickle‐cell thalassemia with splenic sequestration 28959 OTHER DISEASE OF SPLEEN, OTHER D57812 Other sickle‐cell disorders with splenic sequestration 4560 ESOPHAGEAL VARICES W BLEEDING D730 Hyposplenism 4561 ESOPHAGEAL VARICES WO MENTION OF BLEEDING D731 Hypersplenism 45620 ESOPHAGEAL VARICES IN DISEASE CLASSIFIED D732 Chronic congestive splenomegaly ELSEWHERE, W BLEEDING 45621 ESOPHAGEAL VARICES IN DISEASE CLASSIFIED D733 Abscess of spleen ELSEWHERE, WO MENTION OF BLEEDING 5723 PORTAL HYPERTENSION D735 Infarction of spleen 5728 OTHER SEQUELAE OF CHRONIC LIVER DISEASE D7389 Other diseases of spleen 5735 -

Extrarenal Complications of the Nephrotic Syndrome
Kidney International, Vol. 33 (/988), pp. 1184—1202 NEPHROLOGY FORUM Extrarenal complications of the nephrotic syndrome Principal discussant: DAVID B. BERNARD The University Hospital and Boston University Sc/zoo!ofMedicine, Boston, Massachusetts present and equal. The temperature was 100°F. The blood pressure was 110/70 mm Hg in the right arm with the patient supine and standing. The Editors patient had no skin rashes, peteehiae, clubbing, or jaundice. Examina- JORDANJ. COHEN tion of the head and neck revealed intact cranial nerves and normal fundi. Ears, nose, and throat were normal. The jugular venous pressure Jot-IN T. HARRtNOTON was not increased. No lymph glands were palpable in the neck or JEROME P. KASSIRER axillae, and the trachea was midline, cardiac examination was normal. NICOLA05 E. MAmAs Examination of the lungs revealed coarse rales at the right base but no other abnormalities. Abdominal examination revealed aseites, but no Editor abdominal guarding, tenderness, or rigidity. The liver and spleen were Managing not palpable and no masses were present. The urine contained 4± CHERYL J. ZUSMAN protein; microscopic examination revealed free fat droplets, many oval fat bodies, and numerous fatty casts. Five to 10 red blood cells were seen per high-power field, but no red blood cell casts were present. A Universityof'Chicago Pritzker School of Medicine 24-hr urine collection contained 8 g of protein. The BUN was 22 mg/dl; creatinine, 2.0 mg/dl; and electrolytes were and normal. Serum total calcium was 7.8 mg/dl, and the phosphorus was 4.0 Taf is University School of' Medicine mg/dl. -

The Links Between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review
toxins Article The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review Alicja Rydzewska-Rosołowska 1,* , Natalia Sroka 1, Katarzyna Kakareko 1, Mariusz Rosołowski 2 , Edyta Zbroch 1 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (N.S.); [email protected] (K.K.); [email protected] (E.Z.); [email protected] (T.H.) 2 Department of Gastroenterology and Internal Medicine, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 9 December 2020; Published: 11 December 2020 Abstract: The last years have brought an abundance of data on the existence of a gut-kidney axis and the importance of microbiome in kidney injury. Data on kidney-gut crosstalk suggest the possibility that microbiota alter renal inflammation; we therefore aimed to answer questions about the role of microbiome and gut-derived toxins in acute kidney injury. PubMed and Cochrane Library were searched from inception to October 10, 2020 for relevant studies with an additional search performed on ClinicalTrials.gov. We identified 33 eligible articles and one ongoing trial (21 original studies and 12 reviews/commentaries), which were included in this systematic review. Experimental studies prove the existence of a kidney-gut axis, focusing on the role of gut-derived uremic toxins and providing concepts that modification of the microbiota composition may result in better AKI outcomes. Small interventional studies in animal models and in humans show promising results, therefore, microbiome-targeted therapy for AKI treatment might be a promising possibility. -

Renal Papillary Necrosis in Infancy PETER HUSBAND* and K
Arch Dis Child: first published as 10.1136/adc.48.2.116 on 1 February 1973. Downloaded from Archives of Disease in Childhood, 1973, 48, 116. Renal papillary necrosis in infancy PETER HUSBAND* and K. A. HOWLETT From the Departments of Paediatrics and Radiology, Charing Cross Group of Hospitals, Fulham Hospital, London Husband, P., and Howlett, K. A. (1973). Archives of Disease in Childhood, 48, 116. Renal papillary necrosis in infancy. Two infants developed renal papillary necrosis after acute illnesses associated with dehydration. After a short oliguric phase there was a longer phase characterized by impaired urinary concen- tration, hyponatraemia, metabolic acidosis, and a raised blood urea. Intravenous urograms showed contrast collecting in the papillae, with subsequent sinus formation extending into the medulla. In one case impaired urinary concentration was still present 21 months after the initial illness. Renal papillary necrosis was originally described admitted to hospital 9 days later after he had been by Friedreich (1877) in a man aged 70 years. found in his cot, grey, with respiratory distress and a Since then the majority of further cases reported high temperature. 3 days before he had diarrhoea and have also been in adults. It has been thought to vomiting for 24 hours but subsequently tolerated feeds be uncommon and usually to have a fatal outcome of full cream Cow and Gate milk, 180 ml 5 times a day. He was again extremely ill: temperature 40 3 °C, pale, copyright. in infancy and childhood (Davies, Kennedy, and cyanosed with a rapid respiratory rate, but not dehydra- Roberts, 1969). In the newborn infant renal ted. -

Risk of Renal Function Decline in Patients with Ketamine-Associated Uropathy
International Journal of Environmental Research and Public Health Article Risk of Renal Function Decline in Patients with Ketamine-Associated Uropathy Shih-Hsiang Ou 1,2,3, Ling-Ying Wu 4, Hsin-Yu Chen 1,2, Chien-Wei Huang 1,2, Chih-Yang Hsu 1,2, Chien-Liang Chen 1,2 , Kang-Ju Chou 1,2, Hua-Chang Fang 1,2 and Po-Tsang Lee 1,2,* 1 Division of Nephrology, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung 813, Taiwan; [email protected] (S.-H.O.); [email protected] (H.-Y.C.); [email protected] (C.-W.H.); [email protected] (C.-Y.H.); [email protected] (C.-L.C.); [email protected] (K.-J.C.); [email protected] (H.-C.F.) 2 Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan 3 Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan 4 Department of Obstetrics and Gynecology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-7342-2121 (ext. 8090) Received: 8 August 2020; Accepted: 29 September 2020; Published: 4 October 2020 Abstract: Ketamine-associated diseases have been increasing with the rise in ketamine abuse. Ketamine-associated uropathy is one of the most common complications. We investigated the effects of ketamine-associated uropathy on renal health and determined predictors of renal function decline in chronic ketamine abusers. This retrospective cohort study analyzed 51 patients (22 with ketamine- associated hydronephrosis and 29 with ketamine cystitis) from Kaohsiung Veterans General Hospital in Taiwan. -
Path Renal Outline
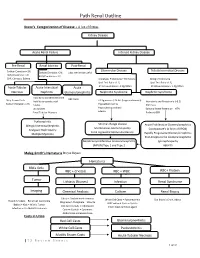
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Acute Tubular Necrosıs After Nephrectomy: Case Presentatıon
Case Report JOJ Case Stud Volume 7 Issue 1 - May 2018 Copyright © All rights are reserved by Ebru Canakci DOI: 10.19080/JOJCS.2018.07.555703 Acute Tubular Necrosıs after Nephrectomy: Case Presentatıon Ebru Canakci1*, Ahmet Karatas2, Ahmet Gultekin1, Zubeyir Cebeci1, Ilker Coskun1 and Anıl Kılınc1 1Department of Anaesthesiology and Reanimation, Ordu University, Turkey 2Department of Internal Medicine & Nephrology, Ordu University, Turkey Submission: May 07, 2018; Published: May 18, 2018 *Corresponding author: Ebru Canakci, Faculty of Medicine, Department of Anaesthesiology and Reanimation, Ordu University, Ordu, Turkey, Email: Abstract ATN, contrary to prerenal azotemia, is not immediately cured upon the recovery of renal perfusion. In its severe form, renal hypoperfusion leads Acute renal failure (ARF) has a clinical presentation with declining renal function and glomerular filtration rate within hours-days. Ischemic trauma, severe hypovolemia, sepsis and severe burns. Acute kidney injury (AKI) is one of the frequently encountered causes of morbidity and mortalityto bilateral in renal hospitals. cortical The necrosis aim of this and study irreversible is to present renal the insufficiency. case with ATN Ischemic after major ATN often surgery develops and subsequent as a result permanent of major surgical kidney injuryintervention, in light of the information from the literature. Keywords: Nephrectomy; Acute Tubular Necrosis; Hemodialysis Introductıon and Objectıve to endogenous or exogenous toxins. Toxins cause intrarenal Acute renal failure (ARF) has a clinical presentation with vasoconstriction, direct tubular toxicity and/or intratubular obstruction and thus lead to ARF [4]. Acute kidney injury (AKI) hours-days. Although there are several differences in the declining renal function and glomerular filtration rate within is one of the frequently encountered causes of morbidity and mortality in hospitals. -

Original Article Thrombocytopenia As a Predictor of Acute Kidney Injury in Patients with Emphysematous Pyelonephritis
Int J Clin Exp Med 2019;12(1):849-854 www.ijcem.com /ISSN:1940-5901/IJCEM0078347 Original Article Thrombocytopenia as a predictor of acute kidney injury in patients with emphysematous pyelonephritis Heng Fan1,3*, Yu Zhao2*, Min Sun1, Jian-Hua Zhu1 1Department of Intensive Care Unit, Ningbo First Hospital, Ningbo, PR China; 2Department of Nephrology, Ningbo Urology and Nephrology Hospital, Ningbo, PR China; 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, PR China. *Equal contributors and co-first authors. Received April 22, 2018; Accepted September 12, 2018; Epub January 15, 2019; Published January 30, 2019 Abstract: Objective: The goal of this study was to investigate the relationship between thrombocytopenia and acute kidney injury (AKI) in patients with emphysematous pyelonephritis (EPN). Methods: A retrospective study was con- ducted in three university hospitals, and early hematological biomarkers were detected to predict the development of AKI in EPN patients. Results: Of 56 EPN patients, 32 (59%) developed AKI. Spearman correlation analysis showed that the nadir platelet count was negatively associated with the peak leukocyte count (r=-0.354, P<0.001), peak serum creatinine (r=-0.429, P<0.001), peak blood urea nitrogen (r=-0.317, P<0.001), and the length of hospital stay (r=-0.442, P<0.001). Furthermore, the area under the receiver-operating-characteristic curve (AU-ROC) was used to evaluate the accuracy of thrombocytopenia for the diagnosis of AKI in EPN patients, and the accuracy of nadir platelet count (AU-ROC: 0.92, 95% CI: 0.85-0.99, P<0.001) predicted AKI was significantly higher than admis- sion platelet count (AU-ROC: 0.84, 95% CI: 0.73-0.94, P<0.001). -

Effects of Bodybuilding Supplements on the Kidney
Ali et al. BMC Nephrology (2020) 21:164 https://doi.org/10.1186/s12882-020-01834-5 RESEARCH ARTICLE Open Access Effects of bodybuilding supplements on the kidney: A population-based incidence study of biopsy pathology and clinical characteristics among middle eastern men Alaa Abbas Ali1, Safaa E. Almukhtar2†, Dana A. Sharif3†, Zana Sidiq M. Saleem4†, Dana N. Muhealdeen1 and Michael D. Hughson1* Abstract Background: The incidence of kidney diseases among bodybuilders is unknown. Methods: Between January 2011 and December 2019, the Iraqi Kurdistan 15 to 39 year old male population averaged 1,100,000 with approximately 56,000 total participants and 25,000 regular participants (those training more than 1 year). Annual age specific incidence rates (ASIR) with (95% confidence intervals) per 100,000 bodybuilders were compared with the general age-matched male population. Results: Fifteen male participants had kidney biopsies. Among regular participants, diagnoses were: focal segmental glomerulosclerosis (FSGS), 2; membranous glomerulonephritis (MGN), 2; post-infectious glomeruonephritis (PIGN), 1; tubulointerstitial nephritis (TIN), 1; and nephrocalcinosis, 2. Acute tubular necrosis (ATN) was diagnosed in 5 regular participants and 2 participants training less than 1 year. Among regular participants, anabolic steroid use was self- reported in 26% and veterinary grade vitamin D injections in 2.6%. ASIR for FSGS, MGN, PIGN, and TIN among regular participants was not statistically different than the general population. ASIR of FSGS adjusted for anabolic steroid use was 3.4 (− 1.3 to 8.1), a rate overlapping with FSGS in the general population at 2.0 (1.2 to 2.8). ATN presented as exertional muscle injury with myoglobinuria among new participants. -

Acute Renal Failure Introduction • Incidence Can Range from 7% to 50
Acute Renal Failure Introduction Incidence can range from 7% to 50% of ICU patients Abrupt decline in kidney function (hours to weeks) Acute Kidney Injury Network Criteria (Bellomo R et al. Crit Care. 2004;8(4):R204-R212) Stage Serum Creatinine Criteria Urine Output 1 Cr inc by 1.5-2x or Cr inc by 0.3 mg/dL <0.5 mL/kg/hr for 6 h 2 Cr inc by 2-3x <0.5 mL/kg/hr for 12 h 3 Cr inc by more than 3x or Cr inc of 0.5 <0.3 mL/kg/hr for 24 hr (or anuria with baseline Cr >4 mg/dL for 12 h) Use criteria after fluid challenge in most cases Causes Pre-renal o Volume depletion o Cardiac o Redistribution o Hepatorenal syndrome . Cirrhosis with ascites . Serum creatinine > 1.5 mg/dL . Not improved by holding diuretics and giving albumin challenge (1g/kg for 2 days) . Absence of shock and nephrotoxins . No intrinsic renal disease i.e. no proteinuria (<500 mg/day) or microhematuria (< 50 RBCs) o NSAIDS o ACE-inhibitor Post-renal o Consider obstruction in every patient with ARF o Sites of obstruction . Bladder neck obstruction . Bilateral ureters o Urine volume is variable. Patients can be asymptomatic and with no change in urine output. o Diagnose with renal USG, straight catherization, or bladder scan Intrinsic o Vascular . Vascular occlusion . Atheroembolic disease Eosinophilia, low complement Can see multi-organ dysfunction, livedo reticularis, blue toes Generally irreversible . Thrombotic microangiopathy Fibrin deposition in the microvasculature, intravascular hemolysis, thrombocytopenia, organ dysfunction Associated disorders: Malignant HTN, HUS/TTP, Scleroderma renal crises, HELLP, drugs (tacrolimus, cyclosporine, mitomycin, Plavix) o Glomerular: RPGN . -

Complement-Mediated Dysfunction of Glomerular Filtration Barrier Accelerates Progressive Renal Injury
BASIC RESEARCH www.jasn.org Complement-Mediated Dysfunction of Glomerular Filtration Barrier Accelerates Progressive Renal Injury Mauro Abbate,* Carla Zoja,* Daniela Corna,* Daniela Rottoli,* Cristina Zanchi,* Nadia Azzollini,* Susanna Tomasoni,* Silvia Berlingeri,* Marina Noris,* Marina Morigi,* and Giuseppe Remuzzi*† *Mario Negri Institute for Pharmacological Research and †Unit of Nephrology and Dialysis, Azienda Ospedaliera Ospedali Riuniti di Bergamo, Bergamo, Italy ABSTRACT Intrarenal complement activation leads to chronic tubulointerstitial injury in animal models of proteinuric nephropathies, making this process a potential target for therapy. This study investigated whether a C3-mediated pathway promotes renal injury in the protein overload model and whether the abnormal exposure of proximal tubular cells to filtered complement could trigger the resulting inflammatory response. Mice with C3 deficiency were protected to a significant degree against the protein overload–induced interstitial inflammatory response and tissue damage, and they had less severe podocyte injury and less proteinuria. When the same injury was induced in wild-type (WT) mice, antiproteinuric treatment with the angiotensin-converting enzyme inhibitor lisinopril reduced the amount of plasma protein filtered, decreased the accumulation of C3 by proximal tubular cells, and protected against interstitial inflammation and damage. For determination of the injurious role of plasma-derived C3, as opposed to tubular cell–derived C3, C3-deficient kidneys were transplanted into WT mice. Protein overload led to the development of glomerular injury, accumulation of C3 in podocytes and proximal tubules, and tubulointerstitial changes. Conversely, when WT kidneys were transplanted into C3-deficient mice, protein overload led to a more mild disease and abnormal C3 deposition was not observed.