Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydronephrosis
Hydronephrosis Natasha Brownrigg RN(EC), MN, NP-Pediatrics Assistant Clinical Professor, McMaster School of Nursing Nurse Practitioner, Pediatric Urology, McMaster Children’s Hospital, Hamilton, ON, Canada Dr. Jorge DeMaria Pediatric Urologist, McMaster Children’s Hospital Professor Department of Surgery/Urology, McMaster University, Hamilton, ON, Canada Dr. Luis H.P. Braga Pediatric Urologist, McMaster Children’s Hospital. Associate professor Department of Surgery/Urology, McMaster University, Hamilton, ON, Canada What is hydronephrosis? Hydro Nephrosis Hydronephrosis += Refers to water Refers to the kidney A build up of fluid or fluid (urine) in the kidney Hydronephrosis is the medical term for a build-up of urine in the kidney. As the urine builds up, it stretches or dilates the inside of the kidney, known as the collecting system. If an unborn baby has hydronephrosis, an ultrasound scan will show a build-up of urine in the kidney. This is called “antenatal hydronephrosis.” Hydronephrosis is found in as many as five out of 100 pregnancies. Hydronephrosis may also be found after birth. For example, if a baby has a urinary tract infection, an ultrasound of the baby’s kidneys and bladder may detect hydronephrosis. Key points to remember if your baby has hydronephrosis • Your baby can grow and develop normally with hydronephrosis. • Hydronephrosis may affect one kidney or both. • Hydronephrosis is a finding, not a disease. • Further tests are needed to find the cause of hydronephrosis. • If the cause is known, a pediatric urologist will discuss the possible treatment. Surgery is sometimes required to correct the cause of the hydronephrosis. Hydronephrosis is often transient and improves without any intervention. -

Acute Phosphate Nephropathy Alexander K
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector http://www.kidney-international.org the renal consult & 2009 International Society of Nephrology Acute phosphate nephropathy Alexander K. Rocuts1, Sushrut S. Waikar1, Mariam P. Alexander1,2, Helmut G. Rennke2 and Ajay K. Singh1 1Renal Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA and 2Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA minute. The rest of the examination was unremarkable. CASE PRESENTATION HerlaboratorydataissummarizedinTable1. A 60-year-old white Latino female with a clinical diagnosis A postoperative kidney ultrasound showed no of diabetes mellitus (diagnosed in 1993) and hypertension hydronephrosis. was referred to the chronic kidney disease clinic at The etiology of the acute kidney injury was unclear. Brigham and Women’s Hospital for the evaluation of acute Progressive diabetic nephropathy exacerbated by other kidney injury; serum creatinine had increased from a contributory factors, such as exposure to lisinopril, baseline of 0.9 to 1.5 mg/dl in a 11-week period. She was acetylsalicylic acid, or naproxen, was regarded as the most asymptomatic at the time of presentation. Her past plausible explanation. Despite discontinuation of these medical history included a total abdominal hysterectomy medications, kidney function did not improve; it worsened with bilateral salpingo oophorectomy and upper in a 4-week period after presentation. Hence, a kidney vaginectomy for high-grade squamous intraepithelial biopsy was performed. lesion of the cervix, 11 weeks prior to presentation. Three weeks prior to presentation (8 weeks after surgery) and within a week of each other, she was evaluated for two consecutive episodes of acute onset of chest pain with pulmonary edema in the setting of severe hypertension. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
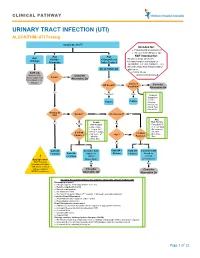
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Obstructive Nephropathy Saulo Klahr
REVIEW ARTICLE Obstructive Nephropathy Saulo Klahr Abstract ages. The incidence of hydronephrosis reported by Bell (1) in a series of32,360 autopsies was 3.8% (3.9% in males, 3.6% in Obstructive nephropathy is a relatively commonentity females). The incidence of clinical manifestations of obstruc- that is treatable and often reversible. It occurs at all ages tive uropathy prior to death was not reported, and it is likely from infancy to elderly subjects. Obstructive uropathy is that hydronephrosis was an incidental finding in many of these classified according to the degree, duration and site of the patients. The incidence of hydronephrosis at autopsy is some- obstruction. It is the result of functional or anatomic le- what lower in children than in adults, being 2%in one series of sions located in the urinary tract. The causes of obstructive 16, 100 autopsies (2). Over 80% of children with hydronephro- uropathy are many. Obstruction of the urinary tract may sis at autopsy were less than 1 year old, with the balance of decrease renal blood flow and the glomerular filtration rate. childhood cases being distributed uniformly through the child- Several abnormalities in tubular function mayoccur in hood years. About 166 patients per 100,000 population had a obstructive nephropathy. These include decreased reab- presumptive diagnosis of obstructive uropathy on admission sorption of solutes and water, inability to concentrate the to hospitals in the United States in 1985 (3). Amongmale pa- urine and impaired excretion of hydrogen and potassium. tients with kidney and urologic disorders, obstructive uropa- Renal interstitial fibrosis is a commonfinding in patients thy ranked fourth at discharge (242 patients/100,000 dis- with long-term obstructive uropathy. -

Renal Tubular Acidosis in Children: State of the Art, Diagnosis and Treatment
www.medigraphic.org.mx Bol Med Hosp Infant Mex 2013;70(3):178-193 REVIEW ARTICLE Renal tubular acidosis in children: state of the art, diagnosis and treatment Ricardo Muñoz-Arizpe,1 Laura Escobar,2 Mara Medeiros3 ABSTRACT Overdiagnosis of renal tubular acidosis (RTA) has been recently detected in Mexican children, perhaps due to diagnostic errors as well as due to a lack of knowledge regarding the pathophysiology and molecular biochemistry involved in this illness. The objective of the present study is to facilitate the knowledge and diagnosis of RTA, a clinical condition infrequently seen worldwide. RTA is an alteration of the acid-base equilibrium due to a bicarbonate wasting in the proximal renal tubules [proximal RTA, (pRTA) or type 2 RTA] or due to a distal nephron hy- drogen ion excretion defect [distal RTA (dRTA) or type 1 RTA]. Hyperkalemic, or type 4 RTA, is due to alterations in aldosterone metabolism. RTA may be primary, secondary, acquired or hereditary and frequently presents secondary to an array of systemic diseases, usually accom- panied by multiple renal tubular defects. The main defect occurs in the transmembrane transporters such as carbonic anhydrase (CA I and + - - + - II), H -ATPase, HCO3 /Cl (AE1) exchanger and Na /HCO3 (NBCe1) cotransporter. Diagnosis should include the presence of hyperchloremic metabolic acidosis with normal serum anion gap (done in an arterial or arterialized blood sample), lack of appetite, polyuria, thirst, growth failure, and rickets; nephrocalcinosis and renal stones (in dRTA); abnormal urine anion gap and abnormal urine/serum pCO2 gradient. Diagnosis of a primary systemic disease must be made in cases of secondary RTA. -

Silent Hydronephrosis, a Hazard Revisited
SILENT HYDRONEPHROSIS, A HAZARD REVISITED By JOEL S. ROSEN, M.D., JOHN B. NANNINGA, M.D. and VINCENT J. O'CONOR, M.D. Midwest Regional Spinal Cord Injury Care System, Chicago, Illinois, U.S.A. Abstract. Six patients with neurogenic bladder secondary to spinal cord injury were seen in our Centre for routine follow-up. All of these individuals had attained the catheter-free state by various means and were, by their standards, functioning very well. They had gone from 6 months to 2t years without genito-urinary re-evaluations. One individual had a normal urogram I year after catheter removal and then 2 years later was noted to have bilateral hydronephrosis. Development of silent hydronephrosis in the catheter-free state, its treatment, and a regimen for following patients are discussed. THE recognition of hydronephrosis following spinal cord injury has been docu mented in the past by several authorities (Talbot & Bunts, 1940; Hutch & Bunts, 1951; Damanski & Gibbon, 1956). Damanski and Gibbon stated that hydro nephrosis occurred in one.;.third of a group of patients suffering from spinal cord injury. The changes have been reported to occur as early as 2 months after injury to as long as 8 years later. Ross (1963) has found hydronephrosis to be associated with the neural damage, urinary infection and back pressure; the importance of each factor varying considerably with the individual, Ascoli and Franch (1970) noted hydronephrosis most frequently in patients more than 4 years post trauma. Warning signs of impending hydronephrosis include the onset of urinary tract infection, deterioration of bladder function, increasing residual urine, bladder hypertonia and the appearance of bladder diverticula and reflux. -

UTI and Pyelonephritis Summary
CHI Franciscan Health UTI & Pyelonephritis Summary UTI/pyelonephritis is one of the most common infectious processes but is also often over-treated. Inappropriate treatment rates vary between 17-26%1,2 and up to 52% in patients with indwelling urinary catheters3. Overtreatment can result in unnecessary medication side effects, the development of Clostridium difficile resistance4, colonization with drug-resistant organisms, and undue costs to the health system. Pharmacists can play and important role in optimizing drug therapy to include the recommendation to stop inappropriate antibiotics when their use is not indicated. Asymptomatic bacteriuria (ASB): the presence of bacteria in the urine without signs/symptoms of infection5 o McGeer Criteria often used to define infection, particularly in the long-term care setting6 Clinical (at least one of the following) Lab (at least one of the following) □ Acute dysuria □ Voided specimen: positive urine culture □ Acute pain, swelling, or tenderness of testes, (> 105 CFU/mL), no more than 2 epididymis, or prostate organisms □ Fever or leukocytosis with one of the following: □ Straight cath specimen: positive urine ○ Acute CVA pain or tenderness culture (> 105 CFU/mL), any number of ○ Suprapubic pain organisms ○ Gross hematuria ○ New or marked increase in incontinence ○ New or marked increase in urgency ○ New or marked increase in frequency □ Two of the preceding symptoms in the absence of fever or leukocytosis o ASB should only be treated in pregnant women and patients undergoing invasive urologic procedures5 o People with ASB also often have WBC in the urine (pyuria) but, in the absence of symptoms, this is not indicative of infection o Caused by: colonization of the bladder or contamination of the sample . -

The Links Between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review
toxins Article The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review Alicja Rydzewska-Rosołowska 1,* , Natalia Sroka 1, Katarzyna Kakareko 1, Mariusz Rosołowski 2 , Edyta Zbroch 1 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (N.S.); [email protected] (K.K.); [email protected] (E.Z.); [email protected] (T.H.) 2 Department of Gastroenterology and Internal Medicine, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 9 December 2020; Published: 11 December 2020 Abstract: The last years have brought an abundance of data on the existence of a gut-kidney axis and the importance of microbiome in kidney injury. Data on kidney-gut crosstalk suggest the possibility that microbiota alter renal inflammation; we therefore aimed to answer questions about the role of microbiome and gut-derived toxins in acute kidney injury. PubMed and Cochrane Library were searched from inception to October 10, 2020 for relevant studies with an additional search performed on ClinicalTrials.gov. We identified 33 eligible articles and one ongoing trial (21 original studies and 12 reviews/commentaries), which were included in this systematic review. Experimental studies prove the existence of a kidney-gut axis, focusing on the role of gut-derived uremic toxins and providing concepts that modification of the microbiota composition may result in better AKI outcomes. Small interventional studies in animal models and in humans show promising results, therefore, microbiome-targeted therapy for AKI treatment might be a promising possibility. -

Pyelonephritis (Kidney Infection)
Pyelonephritis (Kidney Infection) Cathy E. Langston, DVM, DACVIM (Small Animal) BASIC INFORMATION urine collected from the bladder to be negative despite infection in the Description kidney. Abdominal x-rays and an ultrasound may be recommended. Bacterial infection of the kidney is termed pyelonephritis . Infection Although culture of a piece of kidney tissue obtained by biopsy may occur within kidney tissue or in the renal pelvis, the area of increases the chance of finding the infection, the invasiveness of the kidney where urine collects before being transported to the the procedure makes it too risky for general use (the biopsy would bladder. need to be taken from deeper within the kidney than the average Causes kidney biopsy). Contrast x-ray studies, such as an excretory uro- In most cases, a urinary tract infection starts in the bladder and gram (intravenous pyelogram), are sometimes helpful. An excre- the bacteria travel upstream to the kidney. Anything that decreases tory urogram involves taking a series of x-rays after a dye (that the free flow of urine, such as obstruction of the urethra (tube shows up white on x-rays) is given intravenously. Other tests may that carries urine from the bladder to the outside), bladder, ureter be recommended to rule out diseases that cause similar clinical (tube that carries urine from the kidney to the bladder), or kid- signs and other causes of kidney disease. ney, increases the risk that the infection will spread to the kid- ney. The presence of stones and growths in the bladder and kidney TREATMENT AND FOLLOW-UP also increases the risk. -
Urinary Tract Infections
Urinary Tract Infections www.kidney.org Did you know that... n Urinary tract infections (UTIs) are responsible for nearly 10 million doctor visits each year. n One in five women will have at least one UTI in her lifetime. Nearly 20 percent of women who have a UTI will have another, and 30 percent of those will have yet another. Of this last group, 80 percent will have recurrences. n About 80 to 90 percent of UTIs are caused by a single type of bacteria. 2 NATIONAL KIDNEY FOUNDATION n UTIs can be treated effectively with medications called antibiotics. n People who get repeated UTIs may need additional tests to check for other health problems. n UTIs also may be called cystitis or a bladder infection. This brochure answers the questions most often asked about UTIs. If you have more questions, speak to your doctor. What is a urinary tract infection? A urinary tract infection is what happens when bacteria (germs) get into the urinary tract (the bladder) and multiply. The result is redness, swelling and pain in the urinary tract (see diagram). WWW.KIDNEY.ORG 3 Most UTIs stay in the bladder, the pouch-shaped organ where urine is stored before it passes out of the body. If a UTI is not treated promptly, the bacteria can travel up to the kidneys and cause a more serious type of infection, called pyelonephritis (pronounced pie-low-nef-right- iss). Pyelonephritis is an actual infection of the kidney, where urine is produced. This may result in fever and back pain. What causes a UTI? About 80 to 90 percent of UTIs are caused by a type of bacteria, called E. -

Pyonephrosis in Paraplegia
PAPERS READ AT THE 1969 SCIENTIFIC MEETING 121 PYONEPHROSIS IN PARAPLEGIA By J. J. WALSH, M.D., F.R.C.S., M.R.C.P. THIS paper is a report on 32 cases of pyonephrosis treated personally and occurring as a complication of paraplegia or tetraplegia over a period of 17 years from 1950 to 1967. Seven further cases treated by colleagues during that time are not in cluded, so that the incidence would appear to be 39, in a total number of admissions of 3,4580r approximately I per cent. My reason for excluding the 7 cases was that time and circumstance did not allow correlation of information. The criterion for inclusion was the finding of frank pus under tension in the pelvis and calyces of one kidney due to ureteric obstruction. There was no case of simultaneous bilateral pyonephrosis due to this cause. Post-morten findings showing pus in both kidneys as a terminal event in fatal pyelonephritis were not included. Cases showing clear or cloudy urine under tension due to ureteric obstruction were also excluded. It was interesting that a few of the latter cases had longer probable periods of obstruction than one or two of the acceptable pyonephrosis, but had not in fact formed pus. Material. In all there were 32 cases of pyonephrosis 27 male and 5 female. Compared with an admission rate of approximately 23 per cent. of females this would appear to indicate a lower incidence in females but the numbers involved are I feel too small to be significant (Table I). TABLE I Material ! I Average time after onset Average probable period I of obstruction Average age I of spinal lesion 1 (years) (years) (2 excluded) I (days) i I Males 27 1 52.2 9"4 11·6 Females 5 29.2 4.8 7 i Total 32 1 38.8 8·7 II The age ranged from 19 to 65 the average being 38.8 years.