Obstructive Nephropathy Saulo Klahr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
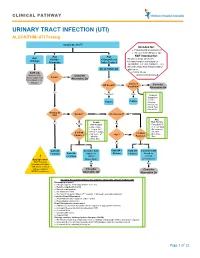
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

Primary Obstructive Megaureter in a Child: a Case Report and Review of Literature
Case Report Annals of Pediatric Research Published: 10 May, 2019 Primary Obstructive Megaureter in a Child: A Case Report and Review of Literature Volkan Sarper Erikci* and Tunahan Altundag Department of Pediatric Surgery, Tepecik Training Hospital, Turkey Abstract Ureterovesical Junction Obstruction (UVJO) is a rare but important cause of hydroureteronephrosis in childhood. A 2-years-old boy suffered from giant hydroureteronephrosis originating from idiopathic ureterovesical junction obstruction. He was treated with excision of narrow ureteric segment with tapering ureteroplasty and a ureteral reimplantation was performed. This case is presented and discussed with reference to etiology of this rather rare anomaly. Keywords: Hydroureteronephrosis; Ureterovesical junction obstruction; Children Introduction Congenital Ureterovesical Junction Obstruction (UVJO) may be observed during fetal age or any stage at the time of childhood. It is aimed in this report to present a male child with obstructive megaureter due to congenital UVJO. He was surgically treated with excision of narrowed distal ureter in addition to tapering ureteroplasty with ureteroneocystostomy. The topic is discussed with special reference to the etiology of this rather rare entity under the light of relevant literature. Case Presentation A 27-months-old boy was admitted to our department with an antenatal history of right Hydroureteronephrosis (HUN). Laboratory tests were otherwise normal except signs of Urinary OPEN ACCESS Tract Infection (UTI) including leucocyturia. -

The Presentation and Management of Neonatal Obstructive Uropathies J
Postgrad Med J: first published as 10.1136/pgmj.48.562.486 on 1 August 1972. Downloaded from Postgraduate Medical Journal (August 1972) 48, 486 -492. The presentation and management of neonatal obstructive uropathies J. H. JOHNSTON F.R.C.S. Alder Hey Children's Hospital, Liverpool Presentation urinary, alimentary and genital tracts share a The existence of a congenital urinary obstruction common evacuatory channel is similarly frequently may be suggested when routine examination of the associated with upper urinary tract lesions. newborn infant reveals such signs as enlargement of one or both kidneys, distension of the bladder or (3) Anomalies of the female genital tract slow, dribbling micturition. The paediatrician should Congenital absence of the vagina is associated also be alerted to the possibility of obstructive uro- with urinary tract anomalies in some 500 of cases pathy when there are present non-urological con- (Bryan, Nigro & Counsellor, 1949). A similar high genital anomalies which often secondarily involve incidence occurs with such conditions as duplication the urinary tract or which are known commonly to ofthe vagina and uterus but these may not be obvious co-exist with congenital urinary tract lesions. clinically in the young child. Secondary involvement, with obstruction, of the urinary tract occurs with space-occupying masses in Deficient abdominal mucsculature (4) by copyright. the pelvis such as hydrometrocolpos or a large intra- Urinary tract anomalies of various degrees of pelvic component of a sacrococcygeal teratoma. are The pelvic tumour, by displacing the bladder, leads severity always present. to chronic urinary retention and upper tract dilata- Cardiac anomalies tion. -

Acute Necrotizing Ureteritis with Obstructive Uropathy Following Instillation of Silver Nitrate in Chyluria: a Case Report
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector C.M. Su, Y.C. Lee, W.J. Wu, et al ACUTE NECROTIZING URETERITIS WITH OBSTRUCTIVE UROPATHY FOLLOWING INSTILLATION OF SILVER NITRATE IN CHYLURIA: A CASE REPORT Chin-Ming Su, Yung-Chin Lee, Wen-Jen Wu, Hung-Lung Ke, Yii-Her Chou, and Chun-Hsiung Huang Department of Urology, Kaohsiung Medical University, Kaohsiung, Taiwan. Chyluria occurs as a result of communication between the lymphatics and the renal pelvis. It is believed that instillation of silver nitrate into the renal pelvis is a safe, minimally invasive and effective treatment for chyluria. We report an unusual complication of acute necrotizing ureteritis following instillation of silver nitrate in a case of chyluria. It resolved completely with non-surgical intervention. The diagnosis and management of chyluria is discussed, with a brief review of the literature. Key Words: chyluria, silver nitrate instillation, necrotizing ureteritis (Kaohsiung J Med Sci 2004;20:512–5) Chyluria occurs as a result of communication between the radiation, or parasite infection were known. The patient lymphatics and the renal pelvis. Its etiology may be classi- underwent cystoscopic examination following a fatty fied as parasitic or non-parasitic. Although the disease is meal. A milky efflux was observed from the right ureteral not life-threatening, it may cause hypoproteinemia, weight orifice. Retrograde pyelography was normal, with no loss, and immunologic disorders due to severe proteinuria pyelolymphatic backflow (Figure 1). Under the diagnosis of [1]. The treatment of chyluria includes limitation of diet to chyluria, 10 mL of 1% silver nitrate was instilled through a mid-chain triglycerides, instillation of silver nitrate, and ureteric catheter. -

Obstructive Uropathy in Children – an Update RANJIT RANJAN ROY1, MD FIROZ ANJUM2, SHAHANA FERDOUS3
BANGLADESH J CHILD HEALTH 2017; VOL 41 (2): 110-116 Obstructive Uropathy in Children – An Update RANJIT RANJAN ROY1, MD FIROZ ANJUM2, SHAHANA FERDOUS3 Abstract: Obstructive nephropathy is a structural or functional hindrance of normal urine flow, sometimes leading to renal dysfunction. Urinary tract obstruction can result from congenital (anatomic) lesion or can be caused by trauma, neoplasia, calculi, inflammation or surgical procedures, although most childhood obstructive lesions are congenital.The clinical features in most of the patients are due to consequences of the obstruction2. Obstruction of the urinary tract generally causeshydronephrosis, which is typically asymptomatic in its early phase. Renal USG gives information about urinary tract dilatation, renal cortical thickness, calyx size, diameter of pelvis, ureter, bladder thickness, tumor & calculi and doppler USG for evaluation of aberrentvessles. Once obstructive nephropathy has been identified therapy focuses on the rapid restoration of normal urine flow either by medical or surgical intervention. Key words: Obstructive Uropathy, Posterior urethral valve, pyelonephritis, IVU, MCU Introduction: urinary tract obstruction impairs renal growth & Urinary tract obstruction refers to as restriction to the development.1 The approach to obstructive urinary outflow that if not managed satisfactorily, leads nephropathy should be to detect site of obstruction, to progressive renal damage. Obstructive uropathy is to find out whether obstruction is complete or partial, an important cause of end stage renal disease unilateral or bilateral, to findthe cause of obstruction requiring renal replacement therapy.1 The kidney is and to decide need forsurgery and to plan the medical an indispensable organ for maintaining homeostasis. treatment.4 The renal system plays diverse role including hormonogenesis, metabolism, detoxification & Etiology: excretion of urine which contains agents that may be Urinary tract obstruction can result from congenital injurious to the body. -
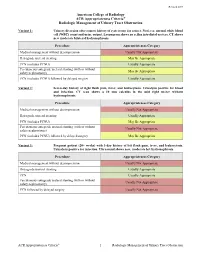
Radiologic Management of Urinary Tract Obstruction
Revised 2019 American College of Radiology ACR Appropriateness Criteria® Radiologic Management of Urinary Tract Obstruction Variant 1: Urinary diversion after remote history of cystectomy for cancer. No fever, normal white blood cell (WBC) count and urine output. Loopogram shows no reflux into distal ureters. CT shows new moderate bilateral hydronephrosis. Procedure Appropriateness Category Medical management without decompression Usually Not Appropriate Retrograde ureteral stenting May Be Appropriate PCN (includes PCNU) Usually Appropriate Percutaneous antegrade ureteral stenting (with or without May Be Appropriate safety nephrostomy) PCN (includes PCNU) followed by delayed surgery Usually Appropriate Variant 2: Seven-day history of right flank pain, fever, and leukocytosis. Urinalysis positive for blood and infection. CT scan shows a 10 mm calculus in the mid right ureter without hydronephrosis. Procedure Appropriateness Category Medical management without decompression Usually Not Appropriate Retrograde ureteral stenting Usually Appropriate PCN (includes PCNU) May Be Appropriate Percutaneous antegrade ureteral stenting (with or without Usually Not Appropriate safety nephrostomy) PCN (includes PCNU) followed by delayed surgery May Be Appropriate Variant 3: Pregnant patient (20+ weeks) with 3-day history of left flank pain, fever, and leukocytosis. Urinalysis positive for infection. Ultrasound shows new, moderate left hydronephrosis. Procedure Appropriateness Category Medical management without decompression Usually Not Appropriate Retrograde ureteral stenting Usually Appropriate PCN Usually Appropriate Percutaneous antegrade ureteral stenting (with or without Usually Not Appropriate safety nephrostomy) PCN followed by delayed surgery Usually Not Appropriate ACR Appropriateness Criteria® 1 Radiologic Management of Urinary Tract Obstruction Variant 4: Advanced cervical carcinoma with decreased estimated glomerular filtration rate <15. Normal WBC, positive pelvic pressure, no flank pain. -

Obstructing Non-Refluxing Megaureter Secondary to Ectopic Ureter: a Rare Case Report
Case Report Obstructing non-refluxing megaureter secondary to ectopic ureter: A rare case report Sanjay P Dhangar1*, Avais A Syed2, Manisha Shengal3, Kalpak Garmade4, Sharyu Gaoture5 1,2Urologist, 3Junior Resident, 4,5Intern, 3-5Dept. of Surgery, 1-5SMBT Institute of Medical Sciences and Research Centre, Nashik, Maharashtra, India *Corresponding Author: Sanjay P Dhangar Email: [email protected] Abstract Megaureter is a nonspecific term implying a spectrum of anomalies associated with grossly dilated diameter of ureter. Ectopic ureter is one such anomaly. Ectopic ureter is defined as any ureter, single or duplex, that does not enter the trigonal area of the bladder. Megaureter is defined as any ureter with a diameter of 7-8 mm2. Megaureter is a common cause of obstructive uropathy among neonates and young children. It is four times more common in boys than in girls and is bilateral in <25% of patients. Ectopic ureter is more common in girls. The left ureter is involved 1.6±4.5 times more often than the right. Treatment of ectopic ureter and megaureter includes exploration, laproscopic or robotic surgery. Ectopic ureter needs ureteric reimplantation and megaureter requires tapering. We present a case of an ectopic ureter leading to secondary obstructing non-refluxing megaureter in a 12 year old female child. Keywords: Ectopic ureter, Megaureter, Ureteric reimplantation, Ureteral tapering. Introduction antibiotics were started. Ultrasound (USG) of abdomen Megaureter is a nonspecific term implying a spectrum of showed left severe hydroureteronephrosis. Contrast anomalies associated with grossly dilated diameter of enhanced computed tomography (CECT) of abdomen (Fig. ureter1. Ectopic ureter is one such anomaly. -

Obstruction of the Urinary Tract 2567
Chapter 540 ◆ Obstruction of the Urinary Tract 2567 Table 540-1 Types and Causes of Urinary Tract Obstruction LOCATION CAUSE Infundibula Congenital Calculi Inflammatory (tuberculosis) Traumatic Postsurgical Neoplastic Renal pelvis Congenital (infundibulopelvic stenosis) Inflammatory (tuberculosis) Calculi Neoplasia (Wilms tumor, neuroblastoma) Ureteropelvic junction Congenital stenosis Chapter 540 Calculi Neoplasia Inflammatory Obstruction of the Postsurgical Traumatic Ureter Congenital obstructive megaureter Urinary Tract Midureteral structure Jack S. Elder Ureteral ectopia Ureterocele Retrocaval ureter Ureteral fibroepithelial polyps Most childhood obstructive lesions are congenital, although urinary Ureteral valves tract obstruction can be caused by trauma, neoplasia, calculi, inflam- Calculi matory processes, or surgical procedures. Obstructive lesions occur at Postsurgical any level from the urethral meatus to the calyceal infundibula (Table Extrinsic compression 540-1). The pathophysiologic effects of obstruction depend on its level, Neoplasia (neuroblastoma, lymphoma, and other retroperitoneal or pelvic the extent of involvement, the child’s age at onset, and whether it is tumors) acute or chronic. Inflammatory (Crohn disease, chronic granulomatous disease) ETIOLOGY Hematoma, urinoma Ureteral obstruction occurring early in fetal life results in renal dys- Lymphocele plasia, ranging from multicystic kidney, which is associated with ure- Retroperitoneal fibrosis teral or pelvic atresia (see Fig. 537-2 in Chapter 537), to various -

Bladder Relaxant Preparations Review 4/20/2009
Bladder Relaxant Preparations Review 4/20/2009 Copyright © 2004 - 2009 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended -

Granulomatous Pyelitis Associated with Urinary Obstruction: a Comprehensive Clinicopathologic Study
Modern Pathology (2006) 19, 1130–1138 & 2006 USCAP, Inc All rights reserved 0893-3952/06 $30.00 www.modernpathology.org Granulomatous pyelitis associated with urinary obstruction: a comprehensive clinicopathologic study Gulfiliz Gonlusen1,2, Anh Truong3, Steven S Shen1,4, Horacio E Adrogue3, Venkat Ramanathan3 and Luan D Truong1,2,3,4 1Department of Pathology, The Methodist Hospital, Houston, TX, USA; 2Department of Pathology, Baylor College of Medicine, Houston, TX, USA; 3Department of Medicine, Renal Section, Baylor College of Medicine, Houston, TX, USA and 4Department of Pathology, Weill Medical College of Cornell University, New York, NY, USA Urinary obstruction is rarely associated with a distinct granulomatous inflammation, which involves the pyelocalyceal system and closely simulates infectious conditions including tuberculosis. Its clinicopathologic features, however, have not been adequately studied since there are only seven isolated reported cases. In a comprehensive study of 112 kidney specimens with urinary obstruction, we identified five cases of granulomatous pyelitis. The features of these cases were detailed and compared with the previously reported cases. Among the five identified subjects, three patients had history of urolithiasis and two had ureteral stenosis and all had stent placement 7 weeks to 12 years before nephrectomy for relief of the unilateral urinary obstruction. The age distribution was between 38 and 81 years. Two had end-stage renal disease or chronic renal failure. The pyelocaliceal system showed frank hydronephrosis (1 case) or partial dilatation (4 cases) and contained cheesy and gritty material in its lumen. Each case showed severe granulomatous inflammation, which was limited to the pelvic wall and closely associated with calcified debris, necrotic inflammatory cells, and material consistent with Tamm–Horsfall protein.