Urinary Antispasmodics TCO 02.2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cigna Home Delivery Pharmacy Drug List
Exclusive Cigna Home Delivery Pharmacy drug list If your prescription drug plan includes Exclusive Cigna Home Delivery Pharmacy, you are required to fill maintenance medication prescriptions (medications taken on an regular basis) through Cigna Home Delivery PharmacySM. Depending on your benefit plan, you are typically allowed three fills before you are required to use Cigna Home Delivery Pharmacy. At that time, if you choose not use Cigna Home Delivery Pharmacy, your plan will not cover the cost of your medication. To see real-time drug pricing, visit myCigna.com and use the Prescription Drug Price Quote tool to search for medications and compare pricing for your selected medications and lower-cost alternatives. The tool provides guidance on options you can discuss with your doctor and shows annual savings when you use Cigna Home Delivery Pharmacy. In addition to cost savings, Cigna Home Delivery Pharmacy offers valuable benefits, such as: • Licensed pharmacists available 24/7 • Up to a 90-day supply of medications in one fill • Standard delivery at no additional cost • Reminders if you forget to fill your prescriptions • Specialty medications, including those that require refrigeration and overnight delivery The following is a list of medications that apply to your Exclusive Cigna Home Delivery Pharmacy plan. Refer to your enrollment materials to understand the medications covered under your plan and to see if there are any requirements such as prior authorization (PA), quantity limits (QL) or Step Therapy (ST), as well as those requiring use of Cigna Home Delivery Pharmacy. If you have questions We’re here to help. Just call us at the toll-free number on your ID card and we will be happy to answer your questions. -

The Effects of Oral Administration of the Novel Muscarinic Receptor
Choi et al. BMC Urology (2020) 20:41 https://doi.org/10.1186/s12894-020-00611-8 RESEARCH ARTICLE Open Access The effects of oral administration of the novel muscarinic receptor antagonist DA- 8010 on overactive bladder in rat with bladder outlet obstruction Jin Bong Choi1, Seung Hwan Jeon2, Eun Bi Kwon3, Woong Jin Bae4, Hyuk Jin Cho2, U-Syn Ha2, Sung-Hoo Hong2, Ji Youl Lee2 and Sae Woong Kim4* Abstract Background: DA-8010 is a novel compound developed for the treatment of overactive bladder (OAB) and urinary incontinence. The aims of this study were to investigate the effects of DA-8010 on OAB in a rat model. Methods: Study animals were divided into the following five groups of seven animals each: a sham-operated control group, a control group with partial bladder outlet obstruction (BOO) (OAB group), and three DA-8010 (doses of 0.3 mg/kg/day, 1 mg/kg/day, and 3 mg/kg/day, respectively) with partial BOO groups. Oral administration of the drugs was continued for 14 days after 2 weeks of partial BOO. After 4 weeks of partial BOO, cystometrography was performed in all groups. Additionally, pro-inflammatory cytokines, Rho-kinases, and histology of the bladder were analyzed. Results: There was a significant increase in the contraction interval and a decrease in contraction pressure in the 3 mg/kg/day DA-8010 group versus those in the OAB group. Rho kinase was also significantly decreased in the DA- 8010 3 mg/kg/day dosage treatment group. The increased ratio of collagen to smooth muscle after partial BOO was significantly attenuated in the DA-8010 3 mg/kg/day dosage group. -

Anticholinergic Drugs Improve Symptoms but Increase Dry Mouth in Adults with Overactive Bladder Syndrome
Source of funding: Evid Based Nurs: first published as 10.1136/ebn.6.2.49 on 1 April 2003. Downloaded from Review: anticholinergic drugs improve symptoms but Health Research Council of Aotearoa increase dry mouth in adults with overactive bladder New Zealand. syndrome For correspondence: Jean Hay-Smith, Hay-Smith J, Herbison P,Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Dunedin School of Cochrane Database Syst Rev 2002;(3):CD003781 (latest version May 29 2002). Medicine, University of Otago, Dunedin, New QUESTION: What are the effects of anticholinergic drugs in adults with overactive Zealand. jean.hay-smith@ bladder syndrome? otago.ac.nz Data sources Parallel arm studies of anticholinergic drugs v placebo for overactive bladder syndrome in Studies were identified by searching the Cochrane adults at 12 days to 12 weeks* Incontinence Group trials register (to January 2002) Weighted event rates and reference lists of relevant papers. Anticholinergic Study selection Outcomes drugs Placebo RBI (95% CI) NNT (CI) Randomised or quasi-randomised controlled trials in Self reported cure or adults with symptomatic diagnosis of overactive bladder improvement (8 studies) 63% 45% 41% (29 to 54) 6 (5 to 8) syndrome, urodynamic diagnosis of detrusor overactiv- RRI (CI) NNH (CI) ity, or both, that compared an anticholinergic drug Dry mouth (20 studies) 36% 15% 138 (70 to 232) 5 (4 to 7) (given to decrease symptoms of overactive bladder) with Outcomes Weighted mean difference (CI) placebo or no treatment. Studies of darifenacin, emepronium bromide or carrageenate, dicyclomine Number of leakage episodes in 24 hours (9 − chloride, oxybutynin chloride, propiverine, propanthe- studies) 0.56 (–0.73 to –0.39) Number of micturitions in 24 hours (8 studies) −0.59 (–0.83 to –0.36) line bromide, tolterodine, and trospium chloride were Maximum cystometric volume (ml) (12 studies) 54.3 (43.0 to 65.7) included. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
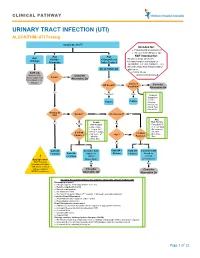
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Urinary Antispasmodics TCO 02.2018
Therapeutic Class Overview Urinary antispasmodics INTRODUCTION • Overactive bladder (OAB) is defined as urinary urgency, with or without urge incontinence, usually with frequency and nocturia, in the absence of a causative infection or pathological conditions. Urinary incontinence has been shown to greatly reduce quality of life in areas such as mental and general health in addition to physical and social functioning (American Urological Association 2019, Coyne et al 2008, Haab 2014, International Continence Society 2015). Children with OAB usually have detrusor overactivity as diagnosed through cystometric evaluation. Neurogenic detrusor overactivity is predominantly caused by a congenital neural tube defect in children (Austin et al 2016, Franco ○ et al 2020). • Behavioral therapies (eg, bladder training, bladder control strategies, pelvic floor muscle training and fluid management) are considered first-line treatment in all patients with OAB (American Urological Association 2019). • Urinary antispasmodics are used as first-line pharmacological therapy in OAB (American College of Obstetricians and Gynecologists 2015, American Urological Association 2019, Blok et al 2020, Burkhard et al 2018). Anticholinergic therapy has been frequently used in patients with neurogenic detrusor overactivity, but there are limited data in this specific population (Haab 2014). • The○ urinary antispasmodics used for the treatment of OAB belong to 2 classes of drugs, which include anticholinergic compounds known as muscarinic receptor antagonists, and the beta-3 adrenergic agonist (AR), mirabegron. The anticholinergic agents act as antagonists of acetylcholine at muscarinic cholinergic receptors, thereby relaxing smooth muscle in the bladder and decreasing bladder contractions. ○ . Oral immediate-release (IR) and extended-release (ER) formulations (LA, XL, and XR) are available for oxybutynin (Ditropan), tolterodine (Detrol), and trospium. -

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

The Role of Antispasmodics in Managing Irritable Bowel Syndrome
DOI: https://doi.org/10.22516/25007440.309 Review articles The role of antispasmodics in managing irritable bowel syndrome Valeria Atenea Costa Barney,1* Alan Felipe Ovalle Hernández.1 1 Internal Medicine and Gastroenterology specialist Abstract in San Ignacio University Hospital, Pontificia Universidad Javeriana, Bogotá, Colombia. Although antispasmodics are the cornerstone of treating irritable bowel syndrome, there are a number of an- tispasmodic medications currently available in Colombia. Since they are frequently used to treat this disease, *Correspondence: [email protected] we consider an evaluation of them to be important. ......................................... Received: 26/10/18 Keywords Accepted: 11/02/19 Antispasmodic, irritable bowel syndrome, pinaverium bromide, otilonium bromide, Mebeverin, trimebutine. INTRODUCTION consistency. The criteria must be met for three consecutive months prior to diagnosis and symptoms must have started Irritable bowel syndrome (IBS) is one of the most fre- a minimum of six months before diagnosis. (3, 4) quent chronic gastrointestinal functional disorders. It is There are no known structural or anatomical explanations characterized by recurrent abdominal pain associated with of the pathophysiology of IBS and its exact cause remains changes in the rhythm of bowel movements with either or unknown. Nevertheless, several mechanisms have been both constipation and diarrhea. Swelling and bloating are proposed. Altered gastrointestinal motility may contribute frequent occurrences. (1) to changes in bowel habits reported by some patients, and a IBS is divided into two subtypes: predominance of cons- combination of smooth muscle spasms, visceral hypersen- tipation (20-30% of patients) and predominance of dia- sitivity and abnormalities of central pain processing may rrhea (20-30% of patients). -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Obstructive Nephropathy Saulo Klahr
REVIEW ARTICLE Obstructive Nephropathy Saulo Klahr Abstract ages. The incidence of hydronephrosis reported by Bell (1) in a series of32,360 autopsies was 3.8% (3.9% in males, 3.6% in Obstructive nephropathy is a relatively commonentity females). The incidence of clinical manifestations of obstruc- that is treatable and often reversible. It occurs at all ages tive uropathy prior to death was not reported, and it is likely from infancy to elderly subjects. Obstructive uropathy is that hydronephrosis was an incidental finding in many of these classified according to the degree, duration and site of the patients. The incidence of hydronephrosis at autopsy is some- obstruction. It is the result of functional or anatomic le- what lower in children than in adults, being 2%in one series of sions located in the urinary tract. The causes of obstructive 16, 100 autopsies (2). Over 80% of children with hydronephro- uropathy are many. Obstruction of the urinary tract may sis at autopsy were less than 1 year old, with the balance of decrease renal blood flow and the glomerular filtration rate. childhood cases being distributed uniformly through the child- Several abnormalities in tubular function mayoccur in hood years. About 166 patients per 100,000 population had a obstructive nephropathy. These include decreased reab- presumptive diagnosis of obstructive uropathy on admission sorption of solutes and water, inability to concentrate the to hospitals in the United States in 1985 (3). Amongmale pa- urine and impaired excretion of hydrogen and potassium. tients with kidney and urologic disorders, obstructive uropa- Renal interstitial fibrosis is a commonfinding in patients thy ranked fourth at discharge (242 patients/100,000 dis- with long-term obstructive uropathy. -

Pharmacy and Poisons Act 1979
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS ACT 1979 1979 : 26 TABLE OF CONTENTS PART I PRELIMINARY 1 Short title 2 Interpretation PART II THE PHARMACY COUNCIL 3 The Pharmacy Council 4 Membership of the Council 4A Functions of the Council 4B Protection from personal liability 4C Annual Report 5 Proceedings of the Council, etc PART III REGISTRATION OF PHARMACISTS 6 Offence to practise pharmacy if not registered 7 Registration as a pharmacist 7A Re-registration as non-practising member 7AA Period of validity of registration 8 Code of Conduct 9 Pharmacy Profession Complaints Committee 10 Investigation of complaint by Committee 10A Inquiry into complaint by Council 10B Inquiry by Council of its own initiative 11 Surrender of registration 12 Restoration of name to register 1 PHARMACY AND POISONS ACT 1979 13 Proof of registration 14 Appeals 14A Fees 14B Amendment of Seventh Schedule 15 Regulations for this part PART IV REGISTRATION OF PHARMACIES 16 Register of pharmacies 17 Registration of premises as registered pharmacies 18 Unfit premises: new applications 19 Unfit premises: registered pharmacies 20 Appeals 21 When certificates of unfitness take effect 22 Regulations for this Part PART V CONTROL OF PRESCRIPTIONS AND IMPORTATION 23 Prescriptions to be in a certain form 23A Validity of a prescription 24 Supply by registered pharmacist of equivalent medicines 25 Restrictions on the importation of medicines 26 Declaration relating to imported medicines [repealed] PART VI CONTROL OF DRUGS 27 Certain substances to be sold on prescription