Bladder Relaxant Preparations
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cigna Home Delivery Pharmacy Drug List
Exclusive Cigna Home Delivery Pharmacy drug list If your prescription drug plan includes Exclusive Cigna Home Delivery Pharmacy, you are required to fill maintenance medication prescriptions (medications taken on an regular basis) through Cigna Home Delivery PharmacySM. Depending on your benefit plan, you are typically allowed three fills before you are required to use Cigna Home Delivery Pharmacy. At that time, if you choose not use Cigna Home Delivery Pharmacy, your plan will not cover the cost of your medication. To see real-time drug pricing, visit myCigna.com and use the Prescription Drug Price Quote tool to search for medications and compare pricing for your selected medications and lower-cost alternatives. The tool provides guidance on options you can discuss with your doctor and shows annual savings when you use Cigna Home Delivery Pharmacy. In addition to cost savings, Cigna Home Delivery Pharmacy offers valuable benefits, such as: • Licensed pharmacists available 24/7 • Up to a 90-day supply of medications in one fill • Standard delivery at no additional cost • Reminders if you forget to fill your prescriptions • Specialty medications, including those that require refrigeration and overnight delivery The following is a list of medications that apply to your Exclusive Cigna Home Delivery Pharmacy plan. Refer to your enrollment materials to understand the medications covered under your plan and to see if there are any requirements such as prior authorization (PA), quantity limits (QL) or Step Therapy (ST), as well as those requiring use of Cigna Home Delivery Pharmacy. If you have questions We’re here to help. Just call us at the toll-free number on your ID card and we will be happy to answer your questions. -

Anticholinergic Drugs Improve Symptoms but Increase Dry Mouth in Adults with Overactive Bladder Syndrome
Source of funding: Evid Based Nurs: first published as 10.1136/ebn.6.2.49 on 1 April 2003. Downloaded from Review: anticholinergic drugs improve symptoms but Health Research Council of Aotearoa increase dry mouth in adults with overactive bladder New Zealand. syndrome For correspondence: Jean Hay-Smith, Hay-Smith J, Herbison P,Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Dunedin School of Cochrane Database Syst Rev 2002;(3):CD003781 (latest version May 29 2002). Medicine, University of Otago, Dunedin, New QUESTION: What are the effects of anticholinergic drugs in adults with overactive Zealand. jean.hay-smith@ bladder syndrome? otago.ac.nz Data sources Parallel arm studies of anticholinergic drugs v placebo for overactive bladder syndrome in Studies were identified by searching the Cochrane adults at 12 days to 12 weeks* Incontinence Group trials register (to January 2002) Weighted event rates and reference lists of relevant papers. Anticholinergic Study selection Outcomes drugs Placebo RBI (95% CI) NNT (CI) Randomised or quasi-randomised controlled trials in Self reported cure or adults with symptomatic diagnosis of overactive bladder improvement (8 studies) 63% 45% 41% (29 to 54) 6 (5 to 8) syndrome, urodynamic diagnosis of detrusor overactiv- RRI (CI) NNH (CI) ity, or both, that compared an anticholinergic drug Dry mouth (20 studies) 36% 15% 138 (70 to 232) 5 (4 to 7) (given to decrease symptoms of overactive bladder) with Outcomes Weighted mean difference (CI) placebo or no treatment. Studies of darifenacin, emepronium bromide or carrageenate, dicyclomine Number of leakage episodes in 24 hours (9 − chloride, oxybutynin chloride, propiverine, propanthe- studies) 0.56 (–0.73 to –0.39) Number of micturitions in 24 hours (8 studies) −0.59 (–0.83 to –0.36) line bromide, tolterodine, and trospium chloride were Maximum cystometric volume (ml) (12 studies) 54.3 (43.0 to 65.7) included. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
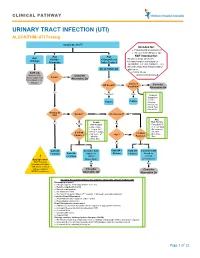
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Urinary Antispasmodics TCO 02.2018
Therapeutic Class Overview Urinary antispasmodics INTRODUCTION • Overactive bladder (OAB) is defined as urinary urgency, with or without urge incontinence, usually with frequency and nocturia, in the absence of a causative infection or pathological conditions. Urinary incontinence has been shown to greatly reduce quality of life in areas such as mental and general health in addition to physical and social functioning (American Urological Association 2019, Coyne et al 2008, Haab 2014, International Continence Society 2015). Children with OAB usually have detrusor overactivity as diagnosed through cystometric evaluation. Neurogenic detrusor overactivity is predominantly caused by a congenital neural tube defect in children (Austin et al 2016, Franco ○ et al 2020). • Behavioral therapies (eg, bladder training, bladder control strategies, pelvic floor muscle training and fluid management) are considered first-line treatment in all patients with OAB (American Urological Association 2019). • Urinary antispasmodics are used as first-line pharmacological therapy in OAB (American College of Obstetricians and Gynecologists 2015, American Urological Association 2019, Blok et al 2020, Burkhard et al 2018). Anticholinergic therapy has been frequently used in patients with neurogenic detrusor overactivity, but there are limited data in this specific population (Haab 2014). • The○ urinary antispasmodics used for the treatment of OAB belong to 2 classes of drugs, which include anticholinergic compounds known as muscarinic receptor antagonists, and the beta-3 adrenergic agonist (AR), mirabegron. The anticholinergic agents act as antagonists of acetylcholine at muscarinic cholinergic receptors, thereby relaxing smooth muscle in the bladder and decreasing bladder contractions. ○ . Oral immediate-release (IR) and extended-release (ER) formulations (LA, XL, and XR) are available for oxybutynin (Ditropan), tolterodine (Detrol), and trospium. -

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

Solifenacin Succinate Tablets PI
465mm (18.31”) 32mm (1.26”) HIGHLIGHTS OF PRESCRIBING • Gastrointestinal Disorders: Use with 3 DOSAGE FORMS AND STRENGTHS Table 1. Percentages of Patients With Identified Adverse Reactions, Derived From Multiple dose studies of solifenacin succinate in elderly volunteers (65 to 80 years) FDA Approved Patient Labeling FDA Approved Patient Labeling INFORMATION caution in patients with decreased Solifenacin succinate tablets are available as follows: All Adverse Events Exceeding Placebo Rate and Reported by 1% or More Patients showed that Cmax, AUC and t1/2 values were 20 to 25% higher as compared to the These highlights do not include gastrointestinal motility (5.3) 5 mg – white, round, standard, normal convex, film-coated, unscored tablets, debossed for Combined Pivotal Studies younger volunteers (18 to 55 years). Solifenacin Succinate Tablets Solifenacin Succinate Tablets all the information needed to • Central Nervous System Effects: with “TV” on one side of the tablet and with “2N” on the other side of the tablet. Placebo Solifenacin Succinate Solifenacin Succinate 8.6 Renal Impairment Read the Patient Information that comes with Read the Patient Information that comes with use SOLIFENACIN SUCCINATE Somnolence has been reported with 10 mg – light-pink to pink, round, standard, normal convex, film-coated, unscored (%) 5 mg (%) 10 mg (%) Solifenacin succinate should be used with caution in patients with renal impairment. TABLETS safely and effectively. solifenacin succinate tablets before you start solifenacin succinate tablets before you start solifenacin succinate. Advise patients not tablets, debossed with “TV” on one side of the tablet and with “3N” on the other side Number of Patients 1216 578 1233 There is a 2.1 fold increase in AUC and 1.6 fold increase in t1/2 of solifenacin in patients See full prescribing information for to drive or operate heavy machinery until of the tablet. -

Solifenacin-Induced Delirium and Hallucinations☆
General Hospital Psychiatry 35 (2013) 682.e3–682.e4 Contents lists available at ScienceDirect General Hospital Psychiatry journal homepage: http://www.ghpjournal.com Case Report Solifenacin-induced delirium and hallucinations☆ Matej Štuhec, Pharm.D. ⁎ Ormoz Psychiatric Hospital, Department for Clinical Pharmacy, Slovenia, Ptujska Cesta 33, Ormoz, Slovenia article info abstract Article history: Solifenacin-induced cognitive adverse effects have not been reported frequently, but solifenacin-induced Received 11 April 2013 delirium and hallucinations with successful switching to darifenacin, without additional drug, have not been Revised 5 June 2013 reported in the literature. In this case report, we present an 80-year-old Caucasian male with insomnia and Accepted 5 June 2013 anxiety symptoms and overactive bladder who developed delirium and hallucinations when treated with Keywords: solifenacin and trazodone. After solifenacin discontinuation and switching to darifenacin, symptoms significantly improved immediately. Such a case has not yet been described in literature; however, an Solifenacin Delirium adverse effect associated with solifenacin can occur, as this report clearly demonstrates. Hallucinations © 2013 Elsevier Inc. All rights reserved. Darifenacin Antimuscarinic adverse effect Case report 1. Introduction tion of Diseases, 10th Revision (ICD-10)], and depression with psychotic features was ruled out with differential diagnosis. Patient reported Solifenacin is a competitive muscarinic receptor antagonist, which insomnia, fear, fatigue, nausea, chest pain, shortness of breath and is used for overactive bladder (OAB) treatment. It acts as an headache. Solifenacin (Vesicare) 5 mg daily in morning dose was antimuscarinic agent, showing the highest affinity for the muscarinic prescribed to him 1 week earlier by his physicians because of OAB. M(3) receptor, which mediates urinary bladder contraction. -

Obstructive Nephropathy Saulo Klahr
REVIEW ARTICLE Obstructive Nephropathy Saulo Klahr Abstract ages. The incidence of hydronephrosis reported by Bell (1) in a series of32,360 autopsies was 3.8% (3.9% in males, 3.6% in Obstructive nephropathy is a relatively commonentity females). The incidence of clinical manifestations of obstruc- that is treatable and often reversible. It occurs at all ages tive uropathy prior to death was not reported, and it is likely from infancy to elderly subjects. Obstructive uropathy is that hydronephrosis was an incidental finding in many of these classified according to the degree, duration and site of the patients. The incidence of hydronephrosis at autopsy is some- obstruction. It is the result of functional or anatomic le- what lower in children than in adults, being 2%in one series of sions located in the urinary tract. The causes of obstructive 16, 100 autopsies (2). Over 80% of children with hydronephro- uropathy are many. Obstruction of the urinary tract may sis at autopsy were less than 1 year old, with the balance of decrease renal blood flow and the glomerular filtration rate. childhood cases being distributed uniformly through the child- Several abnormalities in tubular function mayoccur in hood years. About 166 patients per 100,000 population had a obstructive nephropathy. These include decreased reab- presumptive diagnosis of obstructive uropathy on admission sorption of solutes and water, inability to concentrate the to hospitals in the United States in 1985 (3). Amongmale pa- urine and impaired excretion of hydrogen and potassium. tients with kidney and urologic disorders, obstructive uropa- Renal interstitial fibrosis is a commonfinding in patients thy ranked fourth at discharge (242 patients/100,000 dis- with long-term obstructive uropathy. -

3 Drugs That May Cause Delirium Or Problem Behaviors CARD 03 19 12 JUSTIFIED.Pub
Drugs that May Cause Delirium or Problem Behaviors Drugs that May Cause Delirium or Problem Behaviors This reference card lists common and especially problemac drugs that may Ancholinergics—all drugs on this side of the card. May impair cognion cause delirium or contribute to problem behaviors in people with demena. and cause psychosis. Drugs available over‐the‐counter marked with * This does not always mean the drugs should not be used, and not all such drugs are listed. If a paent develops delirium or has new problem Tricyclic Andepressants Bladder Anspasmodics behaviors, a careful review of all medicaons is recommended. Amitriptyline – Elavil Darifenacin – Enablex Be especially mindful of new medicaons. Clomipramine – Anafranil Flavoxate – Urispas Desipramine – Norpramin Anconvulsants Psychiatric Oxybutynin – Ditropan Doxepin – Sinequan Solifenacin – VESIcare All can cause delirium, e.g. All psychiatric medicaons should be Imipramine – Tofranil Tolterodine – Detrol Carbamazepine – Tegretol reviewed as possible causes, as Nortriptyline – Aventyl, Pamelor Gabapenn – Neuronn effects are unpredictable. Trospium – Sanctura Anhistamines / Allergy / Leveracetam – Keppra Notable offenders include: Insomnia / Sleep Valproic acid – Depakote Benzodiazepines e.g. Cough & Cold Medicines *Diphenhydramine – Sominex, ‐Alprazolam – Xanax *Azelasne – Astepro Pain Tylenol‐PM, others ‐Clonazepam – Klonopin *Brompheniramine – Bromax, All opiates can cause delirium if dose *Doxylamine – Unisom, Medi‐Sleep ‐Lorazepam – Avan Bromfed, Lodrane is too high or -

Magellan Anticholinergic Risk Scale
Magellan Anticholinergic Risk Scale 1 POINT 2 POINTS 3 POINTS GENERIC BRAND GENERIC BRAND GENERIC BRAND Alprazolam Xanax® Amantadine Symmetrel® Amitriptyline Elavil® Aripiprazole Abilify® Baclofen Lioresal® Amoxapine Asendin® Asenapine Saphris® Carbamazepine Tegretol® Atropine -- Captopril Capoten® Carisoprodol Soma® Benztropine Cogentin® Chlordiazepoxide Librium® Cetirizine Zyrtec® Brompheniramine Respa-BR® Chlorthalidone Diuril® Cimetidine Tagamet® Carbinoxamine Arbinoxa® Clonazepam Klonopin® Clidinium & Librax® Chlorpheniramine Chlor-Trimeton® Chlordiazepoxide Clorazepate Tranxene® Cyclizine Cyclivert® Chlorpromazine Thorazine® Codeine -- Cyclobenzaprine Flexeril® Clemastine Tavist® Diazepam Valium® Cyproheptadine Periactin® Clomipramine Anafranil® Digoxin Lanoxin® Disopyramide Norpace® Clozapine Clozaril® Dipyridamole Persantine® Fluphenazine Prolixin® Darifenacin Enablex® Famotidine Pepcid® Loperamide Diamode® Desipramine Norpramin® Fentanyl Duragesic® Loratadine Claritin® Dicyclomine Bentyl® Fluoxetine Prozac® Loxapine Loxitane® Dimenhydrinate Dramamine® Flurazepam Dalmane® Meperidine Demerol® Diphenhydramine Benadryl® Fluvoxamine Luvox® Methocarbamol Robaxin® Doxepin Sinequan® Furosemide Lasix® Oxcarbazepine Trileptal® Flavoxate Urispas® Haloperidol Haldol® Pimozide Orap® Glycopyrrolate Robinul® Hydralazine Apresoline® Prochlorperazine Compazine® Hydroxyzine Atarax® Iloperidone Fanapt® Pseudoephedrine Sudafed® Hyoscyamine Anaspaz® Isosorbide Imdur® Quetiapine Seroquel® Imipramine Tofranil® Mirtazapine Remeron® Trimethobenzamide -

Anticholinergic Drugs and Dementia
DEMENTIA Q&A 24 Anticholinergic drugs and dementia Anticholinergics are a class of drug used to treat a wide variety of medical conditions. As with any medication, anticholinergics can have many beneficial effects, but these need to be balanced against a number of potential risks. This sheet provides information about how these drugs work as well as the impact that they may have in respect to dementia risk and cognitive functioning. What are anticholinergics? in time.4 It has been estimated that in Australia, 33% of people over the age of 65 years take enough Anticholinergics are generally used to inhibit the medications with anticholinergic effects to potentially involuntary movements of muscles or balance the increase their risk of harm.5 The use of anticholinergics production of various chemicals within the body. They is more common in older people because these drugs have been used in treating a wide variety of medical are prescribed for the symptomatic management of conditions including symptoms of psychosis (which medical conditions that often occur in later life. can be caused by bipolar disorder and schizophrenia), depression, urinary incontinence (e.g. overactive How do anticholinergic drugs work? bladder), gastrointestinal spasms, allergies, respiratory Anticholinergics are a class of drug that block the conditions (such as asthma and chronic obstructive action of acetylcholine in the nervous system, a pulmonary disease), Parkinson’s disease, conditions chemical (neurotransmitter) that is used to control concerning muscles in the eye and pupil dilation, messages travelling from one cell to another. They do nausea, sleep disorders and cardiovascular complaints this by blocking the binding of acetylcholine to its (slow heart rhythms) (Table 1).1,2 Some cold and flu receptor in the nerve cells.