Path Renal Outline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Paper Ciliopathies
Eur J Pediatr (2012) 171:1285–1300 DOI 10.1007/s00431-011-1553-z REVIEW Educational paper Ciliopathies Carsten Bergmann Received: 11 June 2011 /Accepted: 3 August 2011 /Published online: 7 September 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cilia are antenna-like organelles found on the (NPHP) . Ivemark syndrome . Meckel syndrome (MKS) . surface of most cells. They transduce molecular signals Joubert syndrome (JBTS) . Bardet–Biedl syndrome (BBS) . and facilitate interactions between cells and their Alstrom syndrome . Short-rib polydactyly syndromes . environment. Ciliary dysfunction has been shown to Jeune syndrome (ATD) . Ellis-van Crefeld syndrome (EVC) . underlie a broad range of overlapping, clinically and Sensenbrenner syndrome . Primary ciliary dyskinesia genetically heterogeneous phenotypes, collectively (Kartagener syndrome) . von Hippel-Lindau (VHL) . termed ciliopathies. Literally, all organs can be affected. Tuberous sclerosis (TSC) . Oligogenic inheritance . Modifier. Frequent cilia-related manifestations are (poly)cystic Mutational load kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous Introduction system, occurring either isolated or as part of syn- dromes. Characterization of ciliopathies and the decisive Defective cellular organelles such as mitochondria, perox- role of primary cilia in signal transduction and cell isomes, and lysosomes are well-known -

Potter Syndrome: a Case Study
Case Report Clinical Case Reports International Published: 22 Sep, 2017 Potter Syndrome: A Case Study Konstantinidou P1,2, Chatzifotiou E1,2, Nikolaou A1,2, Moumou G1,2, Karakasi MV2, Pavlidis P2 and Anestakis D1* 1Laboratory of Forensic and Toxicology, Department of Histopathology, Aristotle University of Thessaloniki, Greece 2Laboratory of Forensic Sciences, Department of Histopathology, Democritus University of Thrace School, Greece Abstract Potter syndrome (PS) is a term used to describe a typical physical appearance, which is the result of dramatically decreased amniotic fluid volume secondary to renal diseases such as bilateral renal agenesis (BRA). Other causes are abstraction of the urinary tract, autosomal recessive polycystic kidney disease (ARPKD), autosomal dominant polycystic kidney disease (ADPKD) and renal hypoplasia. In 1946, Edith Potter characterized this prenatal renal failure/renal agenesis and the resulting physical characteristics of the fetus/infant that result from oligohydramnios as well as the complete absence of amniotic fluid (anhydramnios). Oligohydramnios and anhydramnios can also be due to the result of leakage of amniotic fluid from rupturing of the amniotic membranes. The case reported concerns of stillborn boy with Potter syndrome. Keywords: Potter syndrome; Polycystic kidney disease; Oligohydramnios sequence Introduction Potter syndrome is not technically a ‘syndrome’ as it does not collectively present with the same telltale characteristics and symptoms in every case. It is technically a ‘sequence’, or chain of events - that may have different beginnings, but ends with the same conclusion. Below are the different ways that Potter syndrome (A.K.A Potter sequence) can begin due to various causes of renal failure. They gave numbers to differentiate the different forms, but this system had not caught in the medical and scientific communities [1]. -

Diagnosis and Primary Care Management of Focal Segmental Glomerulosclerosis in Children
1.5 CONTACTCOCONOONNTATACACACT HOURSHOUROURRS 1.5 CONTACT HOURS Monkey Business Images / Thinkstock Diagnosis and primary care management of focal segmental glomerulosclerosis in children Abstract: Focal segmental glomerulosclerosis (FSGS) is a pattern of kidney damage that can occur in individuals at any age, including children. Pediatric patients with FSGS require medication monitoring, growth, and psychological health. This article discusses the NP’s role in the clinical presentation, diagnostic workup, and treatment of FSGS in pediatric patients. By Angela Y. Wong, MS, CPNP-PC and Rita Marie John, DNP, EdD, CPNP-PC, FAANP P, a 10-year-old girl, told her parents several This article discusses the pathophysiology, epide- times, “I feel puffy,” before the family sought miology, clinical presentation, and treatment options J medical attention. This complaint could in- for FSGS. In addition, the article elucidates the role dicate a myriad of issues ranging from sudden weight of the NP in managing this disease when it occurs gain to simple abdominal gas. For this child, “puffy” during childhood. depicted her progressive edema—the only overt symp- tom of her diagnosis of focal segmental glomerulo- ■ Overview sclerosis (FSGS). Although some children with FSGS FSGS is a pattern of kidney damage involving scarring may only present with asymptomatic proteinuria at of glomeruli in the kidney. This pattern of glomerulo- a routine physical, FSGS can affect individuals of all sclerosis is focal and segmental, meaning not all glom- ages, and is a common cause of end-stage renal disease eruli are affected and only parts of the glomeruli are (ESRD) in children.1 damaged, respectively.2 FSGS is the most common Keywords: end-stage renal disease, focal segmental glomerulosclerosis, kidney transplant, nephrotic syndrome, pediatrics 28 The Nurse Practitioner • Vol. -

PATHOLOGY of the RENAL SYSTEM”, I Hope You Guys Like It
ﺑﺴﻢ اﷲ اﻟﺮﺣﻤﻦ اﻟﺮﺣﯿﻢ ھﺬه اﻟﻤﺬﻛﺮة ﻋﺒﺎرة ﻋﻦ إﻋﺎدة ﺗﻨﺴﯿﻖ وإﺿﺎﻓﺔ ﻧﻮﺗﺎت وﻣﻮاﺿﯿﻊ ﻟﻤﺬﻛﺮة زﻣﻼﺋﻨﺎ ﻣﻦ اﻟﺪﻓﻌﺔ اﻟﺴﺎﺑﻘﺔ ٤٢٧ اﻷﻋﺰاء.. ﻟﺘﺘﻮاﻓﻖ ﻣﻊ اﻟﻤﻨﮭﺞ اﻟﻤﻘﺮر ﻣﻦ اﻟﻘﺴﻢ ﺣﺮﺻﻨﺎ ﻓﯿﮭﺎ ﻋﻠﻰ إﻋﺎدة ﺻﯿﺎﻏﺔ ﻛﺜﯿﺮ ﻣﻦ اﻟﺠﻤﻞ ﻟﺘﻜﻮن ﺳﮭﻠﺔ اﻟﻔﮭﻢ وﺳﻠﺴﺔ إن ﺷﺎء اﷲ.. وﺿﻔﻨﺎ ﺑﻌﺾ اﻟﻨﻮﺗﺎت اﻟﻤﮭﻤﺔ وأﺿﻔﻨﺎ ﻣﻮاﺿﯿﻊ ﻣﻮﺟﻮدة ﺑﺎﻟـ curriculum ﺗﻌﺪﯾﻞ ٤٢٨ ﻋﻠﻰ اﻟﻤﺬﻛﺮة ﺑﻮاﺳﻄﺔ اﺧﻮاﻧﻜﻢ: ﻓﺎرس اﻟﻌﺒﺪي ﺑﻼل ﻣﺮوة ﻣﺤﻤﺪ اﻟﺼﻮﯾﺎن أﺣﻤﺪ اﻟﺴﯿﺪ ﺣﺴﻦ اﻟﻌﻨﺰي ﻧﺘﻤﻨﻰ ﻣﻨﮭﺎ اﻟﻔﺎﺋﺪة ﻗﺪر اﻟﻤﺴﺘﻄﺎع، وﻻ ﺗﻨﺴﻮﻧﺎ ﻣﻦ دﻋﻮاﺗﻜﻢ ! 2 After hours, or maybe days, of working hard, WE “THE PATHOLOGY TEAM” are proud to present “PATHOLOGY OF THE RENAL SYSTEM”, I hope you guys like it . Plz give us your prayers. Credits: 1st part = written by Assem “ THe AWesOme” KAlAnTAn revised by A.Z.K 2nd part = written by TMA revised by A.Z.K د.ﺧﺎﻟﺪ اﻟﻘﺮﻧﻲ 3rd part = written by Abo Malik revised by 4th part = written by A.Z.K revised by Assem “ THe AWesOme” KAlAnTAn 5th part = written by The Dude revised by TMA figures were provided by A.Z.K Page styling and figure embedding by: If u find any error, or u want to share any idea then plz, feel free to msg me [email protected] 3 Table of Contents Topic page THE NEPHROTIC SYNDROME 4 Minimal Change Disease 5 MEMBRANOUS GLOMERULONEPHRITIS 7 FOCAL SEGMENTAL GLOMERULOSCLEROSIS 9 MEMBRANOPROLIFERATIVE GLOMERULONEPHRITIS 11 DIABETIC NEPHROPATHY (new) 14 NEPHRITIC SYNDROME 18 Acute Post-infectious GN 19 IgA Nephropathy (Berger Disease) 20 Crescentic GN 22 Chronic GN 24 SLE Nephropathy (new) 26 Allograft rejection of the transplanted kidney (new) 27 Urinary Tract OBSTRUCTION, 28 RENAL STONES 23 HYDRONEPHROSIS -

Glomerulosclerosis in Reflux Nephropathy
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 21(1982), pp. 528—534 NEPHROLOGY FORUM Glomeruloscierosis in reflux nephropathy Principal discussant: RAMzI S. COTRAN Department of Pathology, Brigham and Women's Hospital, Boston, Massachusetts penis, testes, and urethral meatus were normal. The prostate was of normal size. Editors The BUN was 14 mg/dl; creatinine, 1.3 mg/dl (creatinine clearance, 113 mI/mm); blood chemistries were normal; the blood glucose was 93 JORDANJ. COHEN mg/dl; and the complement profile was normal. Serum protein was 7.5 JOHN 1. HARRINGTON gIdI with 4.3 g/dl albumin. Urinalysis showed a pH of 5; a specific JEROME P.KASSIRER gravity of 1.015; 4+ protein, no cells, and no bacteria. Urine culture was sterile. The 24-hour urine protein excretion was 2.4 g. Editor Chest x-ray showed borderline cardiomegaly with clear lungs. An Managing intravenous pyelogram revealed bilateral coarse scarring with caliecta- CHERYL J. ZUSMAN sis. The right kidney was smaller than the left. There was moderate ureterectasia extending down to the ureterovesical junction. A voiding cystourethrogram revealed a large-capacity bladder; the patient had no MichaelReese Hospital and Medical Center urge to void after almost 500 ml of contrast material was instilled. Bilateral reflux was greater and persistent on the left and was intermit- University of Chicago, tent on the right. The left ureter was dilated and tortuous. A left Pritzker School of Medicine ureterocele and right bladder diverticulum were visualized. and A biopsy of the left kidney showed focal scarring with interstitial New England Medical Center fibrosis and chronic inflammation, tubular atrophy, and dilation. -

Potter Syndrome, a Rare Entity with High Recurrence Risk in Women with Renal Malformations – Case Report and a Review of the Literature
CASE PRESENTATIONS Ref: Ro J Pediatr. 2017;66(2) DOI: 10.37897/RJP.2017.2.9 POTTER SYNDROME, A RARE ENTITY WITH HIGH RECURRENCE RISK IN WOMEN WITH RENAL MALFORMATIONS – CASE REPORT AND A REVIEW OF THE LITERATURE George Rolea1, Claudiu Marginean1, Vladut Stefan Sasaran2, Cristian Dan Marginean2, Lorena Elena Melit3 1Obstetrics and Gynecology Clinic 1, Tirgu Mures 2University of Medicine and Pharmacy, Tirgu Mures 3Pediatrics Clinic 1, Tirgu Mures ABSTRACT Potter syndrome represents an association between a specific phenotype and pulmonary hypoplasia as a result of oligohydramnios that can appear in different pathological conditions. Thus, Potter syndrome type 1 or auto- somal recessive polycystic renal disease is a relatively rare pathology and with poor prognosis when it is diag- nosed during the intrauterine life. We present the case of a 24-year-old female with an evolving pregnancy, 22/23 gestational weeks, in which the fetal ultrasound revealed oligohydramnios, polycystic renal dysplasia and pulmonary hypoplasia. The personal pathological history revealed the fact that 2 years before this pregnancy, the patient presented a therapeutic abortion at 16 gestational weeks for the same reasons. The maternal ultra- sound showed unilateral maternal renal agenesis. Due to the fact that the identified fetal malformation was in- compatible with life, we decided to induce the therapeutic abortion. The particularity of the case consists in di- agnosing Potter syndrome in two successive pregnancies in a 24-year-old female, without any significant -

Focal Segmental Glomerulosclerosis in Systemic Lupus Erythematosus
Relato de Caso Focal Segmental Glomerulosclerosis in Systemic Lupus Erythematosus: One or Two Glomerular Diseases? Glomerulosclerose Segmentar e Focal em Lúpus Eritematoso Sistêmico: Uma ou Duas Doenças? Rogério Barbosa de Deus1, Luis Antonio Moura1, Marcello Fabiano Franco2, Gianna Mastroianni Kirsztajn1 1 Nephrology Division and 2 Department of Pathology - Universidade Federal de São Paulo – EPM/UNIFESP- São Paulo. ABSTRACT Some patients with clinical and/or laboratory diagnosis of systemic lupus erythematosus (SLE) present with nephritis which from the morphological point of view does not fit in one of the 6 classes described in the WHO classification of lupus nephritis. On the other hand, nonlupus nephritis in patients with confirmed SLE is rarely reported. This condition may not be so uncommon as it seems. The associated glomerular lesions most frequently described are amyloidosis and focal segmental glomerulosclerosis (FSGS). We report on a 46 year-old, caucasian woman, who fulfilled the American College of Rheumatology criteria for SLE diagnosis: arthritis, positive anti-DNA, ANA, anti-Sm antibodies, and cutaneous maculae. During the follow-up, she presented arthralgias, alopecia, vasculitis, lower extremities edema and decreased serum levels of C3 and C4. Proteinuria was initially nephrotic, but reached negative levels. The serum creatinine varied from 0.7 to 3.0 mg/dl. The patient was submitted to the first renal biopsy at admission and to the second one, 3 years later, with diagnosis of minimal change disease and FSGS, respectively. No deposits were demonstrated by immunofluorescence. In the present case, we believe that the patient had SLE and developed an idiopathic disease of the minimal change disease-FSGS spectrum. -

RENAL BIOPSY and GLOMERULONEPHRITIS by J
Postgrad Med J: first published as 10.1136/pgmj.35.409.604 on 1 November 1959. Downloaded from 604 RENAL BIOPSY AND GLOMERULONEPHRITIS By J. H. Ross, M.D., M.R.C.P. Senior Medical Registrar, The Connaught Hospital, and Assistant, The Mledical Unit, The London Hospital Introduction sufficient to establish a diagnosis in a few condi- Safe methods of percutaneous renal biopsy were tions such as amyloid disease and diabetic first described by Perez Ara (1950) and Iversen glomerulosclerosis, but cortex with at least io and Brun (I95I) who used aspiration techniques. glomeruli is necessary for an accurate assessment Many different methods have since been described; of the renal structure; occasionally, up to 40 the simplest is that of Kark and Muehrcke (1954) glomeruli may be present in a single specimen. who use the Franklin modification of the Vim- The revelation of gross structural changes in Silverman needle. the kidneys of patients who can be recognized Brun and Raaschou (1958) have recently re- clinically as having chronic glomerulonephritis is viewed the majority of publications concerned of little value and does not contribute to manage- with renal biopsy and have concluded that the risk ment or prognosis. In contrast, biopsy specimensProtected by copyright. to the patients is slight. They mentioned three from patients in the early stages of glomerulo- fatalities which have been reported but considered nephritis, particularly those who have developed that, in each case, death was probably due to the the nephrotic syndrome insidiously without disease process rather than the investigation. haematuria or renal failure or who showy no Retroperitoneal haematomata have been recorded evidence of disease other than proteinuria, may in patients with hypertension or uraemia but are provide useful information. -

Glomerulonephritis Management in General Practice
Renal disease • THEME Glomerulonephritis Management in general practice Nicole M Isbel MBBS, FRACP, is Consultant Nephrologist, Princess Alexandra lomerular disease remains an important cause Hospital, Brisbane, BACKGROUND Glomerulonephritis (GN) is an G and Senior Lecturer in important cause of both acute and chronic kidney of renal impairment (and is the commonest cause Medicine, University disease, however the diagnosis can be difficult of end stage kidney disease [ESKD] in Australia).1 of Queensland. nikky_ due to the variability of presenting features. Early diagnosis is essential as intervention can make [email protected] a significant impact on improving patient outcomes. OBJECTIVE This article aims to develop However, presentation can be variable – from indolent a structured approach to the investigation of patients with markers of kidney disease, and and asymptomatic to explosive with rapid loss of kidney promote the recognition of patients who need function. Pathology may be localised to the kidney or further assessment. Consideration is given to the part of a systemic illness. Therefore diagnosis involves importance of general measures required in the a systematic approach using a combination of clinical care of patients with GN. features, directed laboratory and radiological testing, DISCUSSION Glomerulonephritis is not an and in many (but not all) cases, a kidney biopsy to everyday presentation, however recognition establish the histological diagnosis. Management of and appropriate management is important to glomerulonephritis (GN) involves specific therapies prevent loss of kidney function. Disease specific directed at the underlying, often immunological cause treatment of GN may require specialist care, of the disease and more general strategies aimed at however much of the management involves delaying progression of kidney impairment. -

Guidelines on Urolithiasis
Guidelines on Urolithiasis C. Türk (chair), T. Knoll (vice-chair), A. Petrik, K. Sarica, A. Skolarikos, M. Straub, C. Seitz © European Association of Urology 2014 TABLE OF CONTENTS PAGE 1. METHODOLOGY 7 1.1 Introduction 7 1.2 Data identification 7 1.3 Evidence sources 7 1.4 Level of evidence and grade of recommendation 7 1.5 Publication history 8 1.5.2 Potential conflict of interest statement 8 1.6 References 8 2. CLASSIFICATION OF STONES 9 2.1 Stone size 9 2.2 Stone location 9 2.3 X-ray characteristics 9 2.4 Aetiology of stone formation 9 2.5 Stone composition 9 2.6 Risk groups for stone formation 10 2.7 References 11 3. DIAGNOSIS 12 3.1 Diagnostic imaging 12 3.1.1 Evaluation of patients with acute flank pain 12 3.1.2 Evaluation of patients for whom further treatment of renal stones is planned 13 3.1.3 References 13 3.2 Diagnostics - metabolism-related 14 3.2.1 Basic laboratory analysis - non-emergency urolithiasis patients 15 3.2.2 Analysis of stone composition 15 3.3 References 16 4. TREATMENT OF PATIENTS WITH RENAL COLIC 16 4.1 Renal colic 16 4.1.1 Pain relief 16 4.1.2 Prevention of recurrent renal colic 16 4.1.3 Recommendations for analgesia during renal colic 17 4.1.4 References 17 4.2 Management of sepsis in obstructed kidney 18 4.2.1 Decompression 18 4.2.2 Further measures 18 4.2.3 References 18 5. STONE RELIEF 19 5.1 Observation of ureteral stones 19 5.1.1 Stone-passage rates 19 5.2 Observation of kidney stones 19 5.3 Medical expulsive therapy (MET) 20 5.3.1 Medical agents 20 5.3.2 Factors affecting success of medical expulsive -

Guidelines on Urolithiasis
GUIDELINES ON UROLITHIASIS (Text update April 2010) Ch. Türk (chairman), T. Knoll (vice-chairman), A. Petrik, K. Sarica, C. Seitz, M. Straub, O. Traxer Eur Urol 2001 Oct;40(4):362-71 Eur Urol 2007 Dec;52(6):1610-31 J Urol 2007 Dec;178(6):2418-34 Methodology This update is based on the 2008 version of the Urolithiasis guidelines produced by the former guidelines panel. Literature searches were carried out in Medline and Embase for ran- domised control trials and systematic reviews published between January 1st, 2008, and November 30, 2009, and sig- nificant differences in diagnosis and treatment or in evidence level were considered. Introduction Urinary stone disease continues to occupy an important place in everyday urological practice. The average lifetime risk of stone formation has been reported in the range of 5-10%. Recurrent stone formation is a common problem with all types of stones and therefore an important part of the medical care of patients with stone disease. Classification and Risk Factors Different categories of stone formers can be identified based Urolithiasis 251 on the chemical composition of the stone and the severity of the disease (Table 1). Special attention must be given to those patients who are at high risk of recurrent stone formation (Table 2). Table 1: Categories of stone formers Stone composition Category Non-calcium Infection stones: INF stones Magnesium ammonium phosphate Ammonium urate Uric acid UR Sodium urate Cystine CY Calcium First time stone former, without residual So stones stone or stone fragments. First time stone former, with residual Sres stone or stone fragments. -
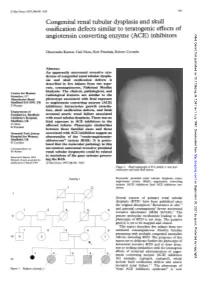
Angiotensin Converting Enzyme (ACE) Inhibitors
J Med Genet 1997;34:541-545 541 Congenital renal tubular dysplasia and skull ossification defects similar to teratogenic effects of J Med Genet: first published as 10.1136/jmg.34.7.541 on 1 July 1997. Downloaded from angiotensin converting enzyme (ACE) inhibitors Dhavendra Kumar, Gail Moss, Rob Primhak, Robert Coombs Abstract An apparently autosomal recessive syn- drome of congenital renal tubular dyspla- sia and skull ossification defects is described in five infants from two sepa- rate, consanguineous, Pakistani Muslim kindreds. The clinical, pathological, and Centre for Human Genetics, 117 radiological features are similar to the Manchester Road, phenotype associated with fetal exposure Sheffield S1O 5DN, UK to angiotensin converting enzyme (ACE) D Kumar inhibitors: intrauterine growth retarda- Department of tion, skull ossification defects, and fetal/ Paediatrics, Sheffield neonatal anuric renal failure associated Children's Hospital, with renal tubular dysplasia. There was no Sheffield, UK fetal exposure to ACE inhibitors in the G Moss affected infants. similarities R Primhak Phenotypic between these familial cases and those Neonatal Unit, Jessop associated with ACE inhibition suggest an Hospital for Women, abnormality of the "renin-angiotensin- Sheffield, UK aldosterone" system (RAS). It is postu- R Coombs lated that the molecular pathology in this Correspondence to: uncommon autosomal recessive proximal Dr Kumar. renal tubular dysgenesis could be related http://jmg.bmj.com/ to mutations of the Received 4 March 1996 gene systems govern- Revised version accepted for ing the RAS. publication 6 March 1997 (JMed Genet 1997;34:541-545) Figure 2 Skull radiograph ofIV.5,family 1: note poor ossification and wide skull sutures.