Angiotensin Converting Enzyme (ACE) Inhibitors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Paper Ciliopathies
Eur J Pediatr (2012) 171:1285–1300 DOI 10.1007/s00431-011-1553-z REVIEW Educational paper Ciliopathies Carsten Bergmann Received: 11 June 2011 /Accepted: 3 August 2011 /Published online: 7 September 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cilia are antenna-like organelles found on the (NPHP) . Ivemark syndrome . Meckel syndrome (MKS) . surface of most cells. They transduce molecular signals Joubert syndrome (JBTS) . Bardet–Biedl syndrome (BBS) . and facilitate interactions between cells and their Alstrom syndrome . Short-rib polydactyly syndromes . environment. Ciliary dysfunction has been shown to Jeune syndrome (ATD) . Ellis-van Crefeld syndrome (EVC) . underlie a broad range of overlapping, clinically and Sensenbrenner syndrome . Primary ciliary dyskinesia genetically heterogeneous phenotypes, collectively (Kartagener syndrome) . von Hippel-Lindau (VHL) . termed ciliopathies. Literally, all organs can be affected. Tuberous sclerosis (TSC) . Oligogenic inheritance . Modifier. Frequent cilia-related manifestations are (poly)cystic Mutational load kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous Introduction system, occurring either isolated or as part of syn- dromes. Characterization of ciliopathies and the decisive Defective cellular organelles such as mitochondria, perox- role of primary cilia in signal transduction and cell isomes, and lysosomes are well-known -

Potter Syndrome: a Case Study
Case Report Clinical Case Reports International Published: 22 Sep, 2017 Potter Syndrome: A Case Study Konstantinidou P1,2, Chatzifotiou E1,2, Nikolaou A1,2, Moumou G1,2, Karakasi MV2, Pavlidis P2 and Anestakis D1* 1Laboratory of Forensic and Toxicology, Department of Histopathology, Aristotle University of Thessaloniki, Greece 2Laboratory of Forensic Sciences, Department of Histopathology, Democritus University of Thrace School, Greece Abstract Potter syndrome (PS) is a term used to describe a typical physical appearance, which is the result of dramatically decreased amniotic fluid volume secondary to renal diseases such as bilateral renal agenesis (BRA). Other causes are abstraction of the urinary tract, autosomal recessive polycystic kidney disease (ARPKD), autosomal dominant polycystic kidney disease (ADPKD) and renal hypoplasia. In 1946, Edith Potter characterized this prenatal renal failure/renal agenesis and the resulting physical characteristics of the fetus/infant that result from oligohydramnios as well as the complete absence of amniotic fluid (anhydramnios). Oligohydramnios and anhydramnios can also be due to the result of leakage of amniotic fluid from rupturing of the amniotic membranes. The case reported concerns of stillborn boy with Potter syndrome. Keywords: Potter syndrome; Polycystic kidney disease; Oligohydramnios sequence Introduction Potter syndrome is not technically a ‘syndrome’ as it does not collectively present with the same telltale characteristics and symptoms in every case. It is technically a ‘sequence’, or chain of events - that may have different beginnings, but ends with the same conclusion. Below are the different ways that Potter syndrome (A.K.A Potter sequence) can begin due to various causes of renal failure. They gave numbers to differentiate the different forms, but this system had not caught in the medical and scientific communities [1]. -

Potter Syndrome, a Rare Entity with High Recurrence Risk in Women with Renal Malformations – Case Report and a Review of the Literature
CASE PRESENTATIONS Ref: Ro J Pediatr. 2017;66(2) DOI: 10.37897/RJP.2017.2.9 POTTER SYNDROME, A RARE ENTITY WITH HIGH RECURRENCE RISK IN WOMEN WITH RENAL MALFORMATIONS – CASE REPORT AND A REVIEW OF THE LITERATURE George Rolea1, Claudiu Marginean1, Vladut Stefan Sasaran2, Cristian Dan Marginean2, Lorena Elena Melit3 1Obstetrics and Gynecology Clinic 1, Tirgu Mures 2University of Medicine and Pharmacy, Tirgu Mures 3Pediatrics Clinic 1, Tirgu Mures ABSTRACT Potter syndrome represents an association between a specific phenotype and pulmonary hypoplasia as a result of oligohydramnios that can appear in different pathological conditions. Thus, Potter syndrome type 1 or auto- somal recessive polycystic renal disease is a relatively rare pathology and with poor prognosis when it is diag- nosed during the intrauterine life. We present the case of a 24-year-old female with an evolving pregnancy, 22/23 gestational weeks, in which the fetal ultrasound revealed oligohydramnios, polycystic renal dysplasia and pulmonary hypoplasia. The personal pathological history revealed the fact that 2 years before this pregnancy, the patient presented a therapeutic abortion at 16 gestational weeks for the same reasons. The maternal ultra- sound showed unilateral maternal renal agenesis. Due to the fact that the identified fetal malformation was in- compatible with life, we decided to induce the therapeutic abortion. The particularity of the case consists in di- agnosing Potter syndrome in two successive pregnancies in a 24-year-old female, without any significant -
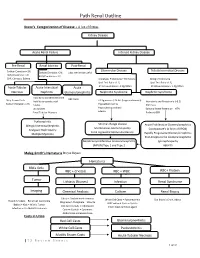
Path Renal Outline
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Meckel–Gruber Syndrome: an Update on Diagnosis, Clinical Management, and Research Advances
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by White Rose Research Online MINI REVIEW published: 20 November 2017 doi: 10.3389/fped.2017.00244 Meckel–Gruber Syndrome: An Update on Diagnosis, Clinical Management, and Research Advances Verity Hartill1,2, Katarzyna Szymanska2, Saghira Malik Sharif1, Gabrielle Wheway3 and Colin A. Johnson2* 1 Department of Clinical Genetics, Yorkshire Regional Genetics Service, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 2 Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, United Kingdom, 3 Faculty of Health and Applied Sciences, Department of Applied Sciences, UWE Bristol, Bristol, United Kingdom Meckel–Gruber syndrome (MKS) is a lethal autosomal recessive congenital anomaly syndrome caused by mutations in genes encoding proteins that are structural or func- tional components of the primary cilium. Conditions that are caused by mutations in ciliary genes are collectively termed the ciliopathies, and MKS represents the most severe condition in this group of disorders. The primary cilium is a microtubule-based organelle, projecting from the apical surface of vertebrate cells. It acts as an “antenna” Edited by: that receives and transduces chemosensory and mechanosensory signals, but also Miriam Schmidts, regulates diverse signaling pathways, such as Wnt and Shh, that have important roles Radboud University Nijmegen, Netherlands during embryonic development. Most MKS proteins localize to a distinct ciliary com- Reviewed by: partment called the transition zone (TZ) that regulates the trafficking of cargo proteins Julia Hoefele, or lipids. In this review, we provide an up-to-date summary of MKS clinical features, Technische Universität München, Germany molecular genetics, and clinical diagnosis. -

16 the Kidney J
16 The Kidney J. Charles Jennette FPO FPO FPO FPO CONGENITAL ANOMALIES IgA Nephropathy (Berger Disease) Renal Agenesis Anti-Glomerular Basement Membrane Ectopic Kidney Glomerulonephritis Horseshoe Kidney ANCA Glomerulonephritis Renal Dysplasia VASCULAR DISEASES CONGENITAL POLYCYSTIC KIDNEY DISEASES Renal Vasculitis Autosomal Dominant Polycystic Kidney Disease Hypertensive Nephrosclerosis (Benign Nephrosclerosis) (ADPKD) Malignant Hypertensive Nephropathy Autosomal Recessive Polycystic Kidney Disease Renovascular Hypertension (ARPKD) Thrombotic Microangiopathy Nephronophthisis–Medullary Cystic Disease Cortical Necrosis ACQUIRED CYSTIC KIDNEY DISEASE DISEASES OF TUBULES AND INTERSTITIUM GLOMERULAR DISEASES Acute Tubular Necrosis (ATN) Nephrotic Syndrome Pyelonephritis Nephritic Syndrome Analgesic Nephropathy Glomerular Inflammation and Immune Mechanisms Drug-Induced (Hypersensitivity) Acute Tubulointerstitial Minimal-Change Glomerulopathy Nephritis Focal Segmental Glomerulosclerosis (FSGS) Light-Chain Cast Nephropathy Membranous Glomerulopathy Urate Nephropathy Diabetic Glomerulosclerosis RENAL STONES (NEPHROLITHIASIS AND Amyloidosis UROLITHIASIS) Hereditary Nephritis (Alport Syndrome) OBSTRUCTIVE UROPATHY AND HYDRONEPHROSIS Thin Glomerular Basement Membrane Nephropathy RENAL TRANSPLANTATION Acute Postinfectious Glomerulonephritis MALIGNANT TUMORS OF THE KIDNEY Type I Membranoproliferative Glomerulonephritis Wilms’ Tumor (Nephroblastoma) Type II Membranoproliferative Glomerulonephritis Renal Cell Carcinoma (RCC) (Dense Deposit Disease) -

Common Paediatric Renal Conditions
Common paediatric renal conditions Few children in South Africa have access to dialysis or renal transplantation, so it is important to recognise kidney disease early enough to prevent progression to end-stage disease. Gertruida van Biljon, MB ChB, MMed(Paed), FCP (Paed) SA, Cert Paediatric Nephrology Professor and Senior Consultant, Department of Paediatrics, Faculty of Health Sciences, University of Pretoria Gertruida van Biljon is a full-time senior consultant in the Department of Paediatrics and Head of the Paediatric Nephrology Unit at Steve Biko Academic Hospital. The Paediatric Renal unit is an accredited paediatric nephrology training unit. Professor Van Biljon has a special interest in the treatment interventions in children with chronic kidney disease, hypertension and nephrotic syndrome. Correspondence to: Gertruida van Biljon ([email protected]) Chronic kidney disease (CKD) is not a Multicystic dysplastic kidney indefinitely as they may have subclinical priority on the health agenda in South (MCDK) evidence of defects in the solitary kidney Africa, and this is particularly true with A MCDK is estimated to occur in and have an increased risk of developing regard to children. Infectious diseases like approximately 1 in 4 000 live births.1 In most hypertension and proteinuria later in life.4 HIV and tuberculosis, respiratory diseases, cases it is discovered by routine antenatal malnutrition and trauma enjoy a far more sonar, which demonstrates a kidney with Posterior urethral valves prominent status as an attention-worthy multiple cysts of varying sizes. The ureter (PUV) (also known as cause of morbidity and mortality. The is atretic and the kidney is non-functional. -

EUROCAT Syndrome Guide
JRC - Central Registry european surveillance of congenital anomalies EUROCAT Syndrome Guide Definition and Coding of Syndromes Version July 2017 Revised in 2016 by Ingeborg Barisic, approved by the Coding & Classification Committee in 2017: Ester Garne, Diana Wellesley, David Tucker, Jorieke Bergman and Ingeborg Barisic Revised 2008 by Ingeborg Barisic, Helen Dolk and Ester Garne and discussed and approved by the Coding & Classification Committee 2008: Elisa Calzolari, Diana Wellesley, David Tucker, Ingeborg Barisic, Ester Garne The list of syndromes contained in the previous EUROCAT “Guide to the Coding of Eponyms and Syndromes” (Josephine Weatherall, 1979) was revised by Ingeborg Barisic, Helen Dolk, Ester Garne, Claude Stoll and Diana Wellesley at a meeting in London in November 2003. Approved by the members EUROCAT Coding & Classification Committee 2004: Ingeborg Barisic, Elisa Calzolari, Ester Garne, Annukka Ritvanen, Claude Stoll, Diana Wellesley 1 TABLE OF CONTENTS Introduction and Definitions 6 Coding Notes and Explanation of Guide 10 List of conditions to be coded in the syndrome field 13 List of conditions which should not be coded as syndromes 14 Syndromes – monogenic or unknown etiology Aarskog syndrome 18 Acrocephalopolysyndactyly (all types) 19 Alagille syndrome 20 Alport syndrome 21 Angelman syndrome 22 Aniridia-Wilms tumor syndrome, WAGR 23 Apert syndrome 24 Bardet-Biedl syndrome 25 Beckwith-Wiedemann syndrome (EMG syndrome) 26 Blepharophimosis-ptosis syndrome 28 Branchiootorenal syndrome (Melnick-Fraser syndrome) 29 CHARGE -

An Amish Founder Variant Consolidates Disruption of CEP55 As a Cause of Hydranencephaly and Renal Dysplasia
European Journal of Human Genetics https://doi.org/10.1038/s41431-018-0306-0 BRIEF COMMUNICATION An Amish founder variant consolidates disruption of CEP55 as a cause of hydranencephaly and renal dysplasia 1,2 1 3 1 1,2 1 Lettie E. Rawlins ● Hannah Jones ● Olivia Wenger ● Myat Aye ● James Fasham ● Gaurav V. Harlalka ● 1 4 1 1 1 Barry A. Chioza ● Alexander Miron ● Sian Ellard ● Matthew Wakeling ● Andrew H. Crosby ● Emma L. Baple 1,2 Received: 25 February 2018 / Revised: 25 September 2018 / Accepted: 7 November 2018 © European Society of Human Genetics 2019 Abstract The centrosomal protein 55 kDa (CEP55 (OMIM 610000)) plays a fundamental role in cell cycle regulation and cytokinesis. However, the precise role of CEP55 in human embryonic growth and development is yet to be fully defined. Here we identified a novel homozygous founder frameshift variant in CEP55, present at low frequency in the Amish community, in two siblings presenting with a lethal foetal disorder. The features of the condition are reminiscent of a Meckel-like syndrome comprising of Potter sequence, hydranencephaly, and cystic dysplastic kidneys. These findings, considered alongside two CEP55 fi 1234567890();,: 1234567890();,: recent studies of single families reporting loss of function candidate variants in , con rm disruption of CEP55 function as a cause of this clinical spectrum and enable us to delineate the cardinal clinical features of this disorder, providing important new insights into early human development. Introduction tumour susceptibility gene 101 (TSG101) and apoptosis- linked gene 2 interacting protein X (ALIX) [4]. The The centrosomal protein 55 kDa (CEP55) is a centro- transcribed CEP55 centrosomal protein has three central some- and midbody-associated protein that has been coiled-coil domains and is expressed at the perinuclear showntoplayacentralrolein cell cycle regulation and membrane, cytoplasm, and nucleus [2, 5]. -

Pediatric Kidney for Radiology Board Exams
Pediatric Kidney for the ABR Core Exam. Matt Covington, MD Listen to the associated podcast episodes available at theradiologyreview.com or on your favorite podcast directory. What is the Potter sequence? Potter sequence results when there is decreased urine output in utero from the kidneys resulting in oligohydramnios which causes potentially fatal hypoplastic lungs, intrauterine growth retardation, characteristic facial abnormalities including a flattened nose and limb abnormalities to include clubbed foot. Although this can occur from any cause of prolonged oligohydramnios, the most classic etiology is bilateral renal agenesis but this could also result from posterior urethral valves or autosomal recessive polycystic kidney disease. What is VACTERL? VACTERL is an association and not a syndrome. Basically, VACTERL describes common congenital anomalies that are all associated with one another. This stands for Vertebral anomalies, Anal anomaly (typically imperforate anus), Cardiac anomalies, TracheaEsophageal fistula or Esophageal atresia, Renal anomalies and Limb anomalies. An interesting fact is that if both limbs are abnormal you can also assume both kidneys are abnormal and if 1 limb is involved, usually only 1 kidney will be involved. VACTERL associations are very high yield for the ABR core exam. What renal abnormality is associated with Caroli syndrome? Caroli syndrome is an autosomal recessive abnormality with classic cystic intrahepatic biliary ductal dilatation consistent with Todani 5 classification. Caroli syndrome is associated with polycystic kidney disease (autosomal dominant or autosomal recessive) as well as medullary sponge kidney. Remember that the central dot sign is classic for Caroli syndrome in which the portal vein is surrounded by the cystic dilated bile ducts. -
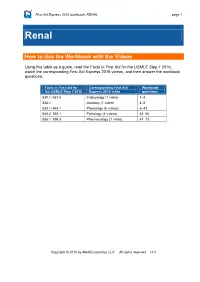
How to Use the Workbook with the Videos
First Aid Express 2016 workbook: RENAL page 1 Renal How to Use the Workbook with the Videos Using this table as a guide, read the Facts in First Aid for the USMLE Step 1 2016, watch the corresponding First Aid Express 2016 videos, and then answer the workbook questions. Facts in First Aid for Corresponding First Aid Workbook the USMLE Step 1 2016 Express 2016 video questions 530.1–531.5 Embryology (1 video) 1–3 532.1 Anatomy (1 video) 4–5 533.1–544.1 Physiology (5 videos) 6–43 544.2–555.1 Pathology (4 videos) 44–66 556.1–559.3 Pharmacology (1 video) 67–73 Copyright © 2016 by MedIQ Learning, LLC All rights reserved v1.0 page 2 First Aid Express 2016 workbook: RENAL Questions EMBRYOLOGY 1. What are the three causes of Potter sequence? (p 530) _________________________________ 2. Which genetic disease is associated with horseshoe kidney? (p 531) _______________________ 3. What error in development occurs that results in unilateral renal agenesis? (p 531) ____________ ______________________________________________________________________________ ANATOMY 4. Why is the left kidney harvested for transplantation rather than the right? (p 532) _____________ ______________________________________________________________________________ 5. Ureters pass ______________ (over/under) the uterine artery and the ductus deferens. (p 532) PHYSIOLOGY 6. Extracellular fluid consists of __________ (high/low) sodium chloride and ___________ (high/low) potassium, whereas intracellular fluid consists of ___________ (high/low) sodium chloride and ___________ (high/low) potassium. (p 533) 7. What is the 60-40-20 rule of total body weight? (p 533) __________________________________ ______________________________________________________________________________ 8. The fenestrated capillary endothelium of the glomerular filtration barrier is responsible for the filtration of plasma by which characteristic: size or charge? (p 533) ________________________________ ______________________________________________________________________________ 9. -

Oligohydramnios Sequence Revisited in Relationship to Arthrogryposis, with Distinctive Skin Changes Judith G
RESEARCH ARTICLE Oligohydramnios Sequence Revisited in Relationship to Arthrogryposis, With Distinctive Skin Changes Judith G. Hall* Departments of Medical Genetics, Pediatrics, University of British Columbia, BC Children’s Hospital Vancouver, British Columbia, Canada Manuscript Received: 3 September 2013; Manuscript Accepted: 1 July 2014 Thirty cases of arthrogryposis associated with longstanding oli- gohydramnios were identified among 2,500 cases of arthrogry- How to Cite this Article: posis (1.2%) and were reviewed for clinical features and natural Hall JG. 2014. Oligohydramnios sequence history.Nonehadrenal agenesis or renal disease. Twenty-twohad revisited in relationship to arthrogryposis, a history of known rupture of membranes. Only 50% had pul- with distinctive skin changes. monary hypoplasia at birth and only two died (7%). Sixty percent (18/30) seemed to have their multiple congenital contractures Am J Med Genet Part A 164A:2775–2792. (MCC) primarily on the basis of compression related to the longstanding oligohydramnios and responded well to physical therapy. On average they did not have intrauterine growth author over a 35-year period. These cases come from clinic visits restriction. “Potter” facies and remarkable skin changes were and consultations, as well as correspondence with physicians and present in all. An excess of males was observed in spite of the lack families. Thirty individuals with longstanding oligohydramnios of genitourinary anomalies. Ó 2014 Wiley Periodicals, Inc. (average five months) were identified. Of