Ige Cross-Reactivity Measurement of Cashew Nut, Hazelnut and Peanuts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -

Some Chemical Composition of Walnut (Juglans Regia L.) Selections from Eastern Turkey
African Journal of Agricultural Research Vol. 5(17), pp. 2379-2385, 4 September, 2010 Available online at http://www.academicjournals.org/AJAR ISSN 1991-637X ©2010 Academic Journals Full Length Research Paper Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey Ferhad Muradoglu1*, H. Ibrahim Oguz2, Kenan Yildiz3 and Hüdai Yilmaz1 1Department of Horticulture, Faculty of Agriculture, Universty of Yuzuncu Yil, 65080 Van, Turkey. 2Professional High School of Kahta, Adiyaman Universty, Adiyaman, Turkey. 3Department of Horticulture, Faculty of Agriculture, Universty of Gaziosmanpaa,Tokat, Turkey. Accepted 10 May, 2010 The aim of this study was to determine the chemical and mineral contents of eighteen walnut genotypes which were newly selected from Hizan (Bitlis) located in Eastern Anatolia. The protein, total fat, total oil (saturated and unsaturated oil) compositions and mineral contents were investigated. It was found that the average value for protein was 18.1% and for total fat was 58.2%. Saturated fatty acids composition values were less than the values of monounsaturated fatty acids composition and polyunsaturated fatty acids composition in all genotypes. Among the identified fatty acids, linoleic acid (50.58 - 66.60%) was the predominant fatty acid followed by oleic acid (14.88 - 28.71%) and linolenic acid (9.16 -16.42%) in all genotypes. The other fatty acids were found in trace contents. The minimum and maximum macronutrient contents of walnut were determined as mg100 g-1 for K (911.0 - 684.3), P (434.7 - 356.2), Ca (756.7 - 388.2), Mg (444.0 - 330.8) and Na (48.9 - 26.1) while minimum and maximum micronutrient contents of walnut were determined for Fe (6.6 - 4.3), Cu (2.8 - 1.8), Mn (5.7 - 2.7) and Zn (4.3 - 2.7). -
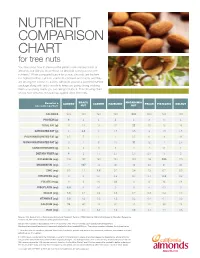
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

AP-42, CH 9.10.2.2: Peanut Processing
9.10.2.2 Peanut Processing 9.10.2.2.1 General Peanuts (Arachis hypogaea), also known as groundnuts or goobers, are an annual leguminous herb native to South America. The peanut peduncle, or peg (the stalk that holds the flower), elongates after flower fertilization and bends down into the ground, where the peanut seed matures. Peanuts have a growing period of approximately 5 months. Seeding typically occurs mid-April to mid-May, and harvesting during August in the United States. Light, sandy loam soils are preferred for peanut production. Moderate rainfall of between 51 and 102 centimeters (cm) (20 and 40 inches [in.]) annually is also necessary. The leading peanut producing states are Georgia, Alabama, North Carolina, Texas, Virginia, Florida, and Oklahoma. 9.10.2.2.2 Process Description The initial step in processing is harvesting, which typically begins with the mowing of mature peanut plants. Then the peanut plants are inverted by specialized machines, peanut inverters, that dig, shake, and place the peanut plants, with the peanut pods on top, into windrows for field curing. After open-air drying, mature peanuts are picked up from the windrow with combines that separate the peanut pods from the plant using various thrashing operations. The peanut plants are deposited back onto the fields and the pods are accumulated in hoppers. Some combines dig and separate the vines and stems from the peanut pods in 1 step, and peanuts harvested by this method are cured in storage. Some small producers still use traditional harvesting methods, plowing the plants from the ground and manually stacking them for field curing. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -
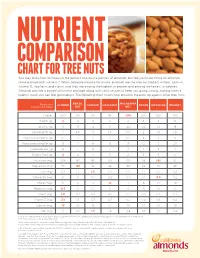
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Canarium Ovatum) Shells
International Journal of Engineering Research And Management (IJERM) ISSN: 2349- 2058, Volume-04, Issue-09, September 2017 Physical and Mechanical Properties of Particleboard Utilizing Pili Nut (Canarium Ovatum) Shells Ariel B. Morales are widely used for various purposes. It is a lightweight and Abstract— This study aims to make a 200 mm x 200 mm strong kind of plastic that can be easily remolded into particleboard with nine millimeter thickness using Crushed Pili different shapes [5]. This study also helps in lessening Nut Shells (CPNS) and sawdust as raw materials with the ratios non-biodegradable wastes and be useful as a resin. This study (CPNS: sawdust) 100:0, 75:25, and 50:50 by weight and determine their physical and mechanical properties. Also, the specifically aims the following objectives: results were compared to the standard properties of commercial • To determine its physical property such as density, unit particleboard set classified by the Philippine Standard weight and thickness swelling, Association (PHILSA). The raw materials were mixed with • To determine the mechanical property, specifically High-Density Polyethylene (HDPE) as an adhesive. All Modulus of Rupture (MOR). particleboards have passed and exceeded the PHILSA standard • To compare the results to the standard properties for for particle board where 100:0 mixtures with density of 1204.09 kg/m3 has the highest MOR of 110 MPa and lowest thickness commercial particleboard from Philippine Standard swelling of 1.11%. Therefore, particleboard made from Pili nut Association (PHILSA) if it will pass or exceed the given shells has an outstanding property compared to commercial standards. particleboards that depends on its application. -

Almond Cashew Barfi Recipe
150 Jackson St, Petone T: 568 4149 E: [email protected] ALMOND CASHEW BARFI RECIPE Almond Walnut Cashew Barfi is a healthy, quality substitute for candy that your entire family will enjoy Ingredients Makes 24 pcs 1/2 c almonds 1/2 c water 1/2 c walnuts 1/2 tsp cardamom powder 1/2 c cashew nuts 1 Tbsp sliced almonds to garnish 1 1/4 c sugar Method 1. Dry grind the walnuts, cashews and almonds in a food processor. 2. Dry roast the grounded nuts in a frying pan on low medium heat. 3. Roast them just enough so that the nuts start to give off an aroma. It will take about 4 to 5 minutes. Remove from heat and set aside. 4. Put the sugar and water together in a saucepan on medium heat. Bring to a boil to make the 1 thread syrup or on the candy thermometer it should reach 230 degrees F. 5. Turn off the heat and stir in the cardamom powder. 6. Add the nuts to the syrup and mix, and then spread over a greased 8-inch plate. Note: don’t let the syrup cool off. It must be spread while still hot. 7. Wait a few minutes until barfi is set but still soft. 8. Then cut the barfi into any shape you like (such as square, diamond, triangle). 9. Garnish each piece of barfi with sliced almonds while the barfi is still soft. 10. Allow the barfi to cool for about an hour to dry and hold its shape. -

Trailer Decontamination)
Preventing Peanut Cross-Contamination in Georgia Pecans (Trailer Decontamination) Peanut cross contamination within the pecan industry is a serious concern. One potential source of cross-contamination within the Georgia pecan industry is the use of trailers previously used in peanut harvest for transport of pecans during pecan harvest. Sixty three percent of all food-allergen related deaths in the U.S. are the result of peanut allergies. These allergies result from allergenic proteins found in the peanut kernel. If pecan growers were to use trailers which previously held peanuts during pecan harvest, the potential would exist for the pecans to be contaminated with peanut allergens through peanut oil residue left on the surface of the trailer or through left-over peanuts still remaining in the trailer from peanut harvest. If contaminated pecans were to be processed in a shelling plant, the entire shelling plant itself could become contaminated. Ultimately, a peanut allergin-related death traced back to the consumption of peanut contaminated pecans could have a devastating effect upon the Georgia pecan industry. Therefore, before pecans are dumped into trailers at harvest, the following steps are suggested for decontaminating trailers that may have previously been used in peanut harvest: 1. Clean & sweep trailer thoroughly, assuring that all previous products are removed from trailer. 2. Wash trailer with soap & water solution, making sure all surfaces are scrubbed well with a brush. Use any FDA approved food grade detergents. 3. Rinse trailer out thoroughly with water making sure there are no visual signs of cleaning solution left inside. 4. Sanitize inside of trailer with at least 50 ppm chlorine water solution. -

BAY AREA MENU Seasonal Items in Green Contains Gluten Vegan
BAY AREA MENU seasonal items in green contains gluten vegan Before placing your order, please inform your server if a person in your party has a food allergy. SEASONALS FALAFEL + FETA SPRING CHICKEN UMAMI GRAIN BOWL broccoli leaf, chopped organic mesclun, shredded organic quinoa + farro, romaine, roasted beets, kale, roasted zucchini + swiss chard, pea shoots, spicy broccoli, parsley, yellow squash + asparagus, red onion, spicy sunflower mint, local feta, baked basil, shredded carrots, seeds, roasted sesame falafel, lemon garlic chili parmesan crisps, roasted tofu, roasted portobello vinaigrette chicken, pesto vinaigrette mushrooms, miso ginger 460 cal $9 sesame dressing 480 cal $11.25 615 cal $10.25 GREENS SOUPS KALE CAESAR OMG OMEGA GUACAMOLE GREENS ORGANIC LENTIL CHICKPEA shredded kale, chopped organic arugula, organic organic mesclun, tomatoes, small 160 cal $3.50 romaine, tomatoes, shaved baby spinach, cucumbers, red onion, tortilla chips, large 240 cal $5.50 parmesan, parmesan crisps, tomatoes, basil, avocado, avocado, roasted chicken, roasted chicken, fresh lime nori furikake, roasted fresh lime squeeze, lime squeeze, caesar dressing steelhead, miso sesame cilantro jalapeño vinaigrette ginger dressing 430 cal $9.75 540 cal $10.75 550 cal $13.25 HUMMUS TAHINA RAD THAI SPICY SABZI BEVERAGES shredded kale, chopped organic arugula, organic organic baby spinach, $2.50 romaine, tomatoes, mesclun, bean sprouts, shredded kale, spicy red onion, cucumbers, carrots, shredded cabbage, quinoa, spicy broccoli, HIBISCUS LIME FRESCA pita chips, -

Wholesome and Organic
1 3231 Camino de los Coches #107 Carlsbad, CA. 92009 Tel: 760-230-9282 Instagram/FB: @bigfootnaturalcafe Organic – Vegan – Wholesome – GF Available Wholesome and Organic Pasta and Rice: Mac N’ Cheez (GF) $13.75 (GF macaroni, cauliflower, broccoli, carrots cooked with house broth and house made cashew cheez sauce) Cajun Dirty Mac (GF by request) $13.75 (GF mac with roasted veggies, mushrooms, black beans and tempeh bits, in a house made creamy Creole sauce packed with leafy greens) Woodland Rice Bowl (GF) $15.25 (Sprouted brown rice, grilled portabella mushrooms, black beans, roasted sweet potatoes, grilled zucchini and avocado, drizzled with house cheez sauce and mole sauce (chocolate free).) Upgrades: Add Avocado $2.50 or house made super GF bread $2.50 Hand Crafted Tacos and Burritos: Chili Guava infused Jackfruit Tacos (GF) $13.75 (2 tacos with chili-guava infused jackfruit, slaw, maple toasted coconut chips, avocado and cashew sauce. Served with a small side of sweet potato and black bean salad) Kung Pao Chickpea Tacos (GF) $13.75 (2 tacos with roasted kung pao chickpeas, cashews and shaved brussel sprouts , red slaw, maple toasted coconut chips, avocado and kung pao sauce. Served with a small side of sweet potato and black bean salad) Tiger Stripe Burrito $14.25 (GF Option Available) (Blackened tempeh, sprouted brown rice, sweet potatoes, black beans wrapped in a warm whole wheat tortilla and smothered in our house cheez and mole sauce. Served with a side salad.) Jaka Asada Burrito $15.25 (Rosemary potatoes, chili-guava infused jackfruit (Jaka), portabella mushrooms, soaked chickpeas, hemp hearts all tossed in our Mole made with Modern Times Blackhouse Coffee Stout, wrapped in a warm whole wheat tortilla and smothered in our house cheez sauce. -

Comparative Proteomic Analysis of Walnut (Juglans Regia L.) Pellicle Tissues Reveals the Regulation of Nut Quality Attributes
life Article Comparative Proteomic Analysis of Walnut (Juglans regia L.) Pellicle Tissues Reveals the Regulation of Nut Quality Attributes Paulo A. Zaini 1, Noah G. Feinberg 1, Filipa S. Grilo 2 , Houston J. Saxe 1 , Michelle R. Salemi 3, Brett S. Phinney 3 , Carlos H. Crisosto 1 and Abhaya M. Dandekar 1,* 1 Department of Plant Sciences, University of California, Davis, CA 95616, USA; [email protected] (P.A.Z.); [email protected] (N.G.F.); [email protected] (H.J.S.); [email protected] (C.H.C.) 2 Department of Food Sciences and Technology, University of California, Davis, CA 95616, USA; [email protected] 3 Proteomics Core Facility, University of California, Davis, CA 95616, USA; [email protected] (M.R.S.); [email protected] (B.S.P.) * Correspondence: [email protected] Received: 2 November 2020; Accepted: 25 November 2020; Published: 27 November 2020 Abstract: Walnuts (Juglans regia L.) are a valuable dietary source of polyphenols and lipids, with increasing worldwide consumption. California is a major producer, with ‘Chandler’ and ‘Tulare’ among the cultivars more widely grown. ‘Chandler’ produces kernels with extra light color at a higher frequency than other cultivars, gaining preference by growers and consumers. Here we performed a deep comparative proteome analysis of kernel pellicle tissue from these two valued genotypes at three harvest maturities, detecting a total of 4937 J. regia proteins. Late and early maturity stages were compared for each cultivar, revealing many developmental responses common or specific for each cultivar. Top protein biomarkers for each developmental stage were also selected based on larger fold-change differences and lower variance among replicates, including proteins for biosynthesis of lipids and phenols, defense-related proteins and desiccation stress-related proteins.