Functional Neuromuscular Electrical Stimulation 8.03.01
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dive Theory Guide
DIVE THEORY STUDY GUIDE by Rod Abbotson CD69259 © 2010 Dive Aqaba Guidelines for studying: Study each area in order as the theory from one subject is used to build upon the theory in the next subject. When you have completed a subject, take tests and exams in that subject to make sure you understand everything before moving on. If you try to jump around or don’t completely understand something; this can lead to gaps in your knowledge. You need to apply the knowledge in earlier sections to understand the concepts in later sections... If you study this way you will retain all of the information and you will have no problems with any PADI dive theory exams you may take in the future. Before completing the section on decompression theory and the RDP make sure you are thoroughly familiar with the RDP, both Wheel and table versions. Use the appropriate instructions for use guides which come with the product. Contents Section One PHYSICS ………………………………………………page 2 Section Two PHYSIOLOGY………………………………………….page 11 Section Three DECOMPRESSION THEORY & THE RDP….……..page 21 Section Four EQUIPMENT……………………………………………page 27 Section Five SKILLS & ENVIRONMENT…………………………...page 36 PHYSICS SECTION ONE Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through the glass of the mask and the air space, the difference in speed causes the light rays to bend; this is called refraction. To the diver wearing a normal diving mask objects appear to be larger and closer than they actually are. -
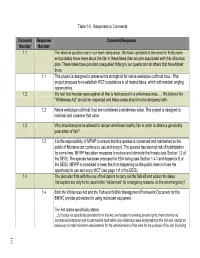
Final EIS Responses to Comments 1-40
Table 1-2. Responses to Comments Comment Response Comment/Response Number Number 1.1 The lakes in question are in our main camp area. We have operated in this area for thirty years and probably know more about the fish in these lakes than anyone associated with this ridiculous plan. These lakes have provided unequalled fishing to our guests and all others that have fished them. 1.1 This project is designed to preserve this stronghold for native westslope cutthroat trout. This project proposes to re-establish WCT populations in all treated lakes, which will maintain angling opportunities. 1.2 We feel that his plan goes against all that is held sacred in a wilderness area. … We believe the "Wilderness Act" should be respected and these areas should not be tampered with. 1.2 Native westslope cutthroat trout are considered a wilderness value. This project is designed to maintain and conserve that value. 1.3 Why should anyone be allowed to tamper with these healthy fish in order to obtain a genetically pure strain of fish? 1.3 It is the responsibility of MFWP to ensure that this species is conserved and maintained so the public of Montana can continue to use and enjoy it. The species has been at risk of hybridization for some time. MFWP has taken measures to reduce and eliminate the threats (see Section 1.2 of the DEIS). The species has been proposed for ESA listing (see Section 1.4.1 and Appendix B of the DEIS). MFWP is mandated to keep this from happening so the public does not lose the opportunity to use and enjoy WCT (see page 1-8 of the DEIS). -

Quality Rental Tools and Equipment– Well Serviced, Priced Right
RENTAL CATALOG QUALITY RENTAL TOOLS AND EQUIPMENT– WELL SERVICED, PRICED RIGHT. RENTAL INDEX CORE DRILLS SAFETY AIR COMPRESSORS Core Drills . 1 Confined Space Tripod . 13 Air Compressors - Tow Behind . 24 Accessories . 1 Davit System . 13 Air Compressors - Electric . 24 Bits . 2 Fall Protection Carts . 13 Retractable Lifelines . 13 BREAKERS ELECTRICAL Horizontal Lifelines . 13 Air (30lb, 60lb and 90lb) . 25 Cable Cutters . 3 Gas Monitors . 13 Electric . 25 Cable Crimpers . 3 Monitor Pumps . 13 Air Hose . 25 Cable Pullers . 3 Cable Sheaves . 3 BLOWERS BAKER SCAFFOLD Pull Monitor . 3 Blowers . 14 Baker Scaffold . 25 Cable Feeder . 5 Reel Stands . 5 MATERIAL LIFTS AIR TOOLS Reel Stand Spindles . 5 Material Lifts . 14 Rivet Buster . 26 Rod and Strut Cutters . 5 Roustabouts . 14 Rivet Buster Bits . .26 Conduit Benders . 5-6 A/C Lift and Mover . 14 Scalers . 26 Conduit Cart . 6 Gantry . .15 Chippers . 26 Knock Out Sets . 6 Bits for Air Chippers . 26 Cable Stripper . 6 HYDRAULIC CYLINDERS Impacts . 26 Hydraulic Cylinders/Bags . 15-16 Air Hose . 26 RADIOS Hydraulic Pumps and Hoses . 16 Saws . 28 Radios . 6 TORQUE TOOLS COMPACTION PIPING TOOLS Hand Torque Wrenches . 16 Plate Compactor . .28 Threaders . 7 Cordless Torque Wrenches . 18 Jumping Jack Tampers . 28 Threader Accessories . 7 Hydraulic Torque Wrenches . 18 Roll Groovers . 7 Punches . 18 GENERATORS AND PUMPS Groover Accessories . 9 Generators . 28 See Snake Camera . 9 CHAIN HOISTS Gas Pumps . 29 Locators . 9 Hand Chainfall Hoist . 18-19 Electric Pumps . 29 Pipe Freeze Kits . 9 Hand Lever Hoist (Come-A-Long) . 19 Pipe Thawers . 9 Electric Chainfalls . 20-21 WELDING T-Drills . 9 Air Hoist . -

Diving Incidents Report Foreward Introduction
APPENDIX A - DIVING INCIDENTS REPORT FOREWARD The 1992 Diving Incidents Report, produced by The British Sub-Aqua Club (BSAC) in the interest of promoting diving safety. As the Goveming Body of the sport of sub-aqua diving within the United Kingdom, the BSAC publishes this information and makes it freely available, subject to the bounds of medical confidentiality, for the benefit of all those concerned with the organisation and conduct of sport diving activities, and in particular, all divers. The BSAC Incidents Reporting Scheme has been established for some 27 years, longer than some diver training organisations. It uses information gathered from a large variety of sources, including the individuals and clubs involved, H.M. Coastguard, recompression chamber operators, the Institute of Naval Medicine and a press cuttings service. All reports received are analysed and summarised in this report with the intention of highlighting any lessons that can be learnt. The BSAC uses this information to identify any trends in diving incidents in order togive its best advice and, if necessary, introduce new training prior to these trends becoming commonplace occurrences. This year (1992) there have been 123 reports received, and it is estimated that over 1.5 million 'man-dives' have been carried out. This is an apparent 30% reduction in dives carried out when compared to 1991 ,possibly due to both the depressed state of the economy and due to the inclement weather on the South Coast for most of the year. The vast majority of these dives were carried out in complete safety and attracted no publicity. -

Dock Leveler Air-Air-Pair-Poweredpowered
DOCK LEVELER AIR-AIR-PAIR-POWEREDPOWERED 1 aFX® DOCK LEVELER PRODUCT DETAILS Provides better stress distribution, resulting in less wear and It’s the original Kelley air-powered dock leveler that revolutionized the industry. In addition to delivering a safe, powered 1. Unique Lambda Beam Design: fatigue, longer life and less downtime for emergency maintenance. performance at a cost comparable to mechanical dock levelers, the aFX features airDefense® — preventing stump-out and providing a measure of free fall protection. The aFX is also backed by the strongest warranty in the industry, with a rated lifetime lip hinge 2. Self-Cleaning, Lug-Type Hinge: With heavy lugs welded directly to each beam and lip warranty, 10-year structural warranty and a 5-year parts and labor warranty on the lifting system, making it the preferred choice of assembly, the Kelley lug-style hinge design is the strongest in the industry. warehousing operations worldwide. 3. Durable, Reinforced Composite Lifting Bag: Lifting bag with RF welded, reinforced polyvinyl chloride coated polyester fibers operates without loss of performance in temperatures from -65°F to +200°F, even if punctured. The airbag cannot be over-inflated (maximum motor pressure is 4 PSI), and has been tested for chemical immersion and rodent damage. 2 4. Deck Support Legs with airDefense®: High efficiency 60,000 lbs. dock level support legs 9 are drop tested to the full rated capacity of the dock leveler. Special airDefense rollers glide along a reinforced cam, preventing stump-out and providing fluid, free-float motion, along with a measure of free fall protection in the event of premature trailer separation. -

2 & 3 Teir Lifting Bags
2 & 3 TEIR LIFTING BAGS CONTENTS 3 2 & 3 TEIR LIFTING BAGS 5 STANDARD REGULATOR 6 ALUMINIUM CONTROLLERS 7 HANDHELD CONTROLLERS 8 HOSES 2 & 3 TIER LIFTING BAGS MFC International offer a 2 or 3 tier low pressure airlift These bags have a large contact area which means they bag designed primarily for the recovery of vehicles. The uniformly distribute lift pressure across a large surface area, tiered design allows for greater stability during the lift than making them suitable for lifting at weak points of a vehicle such conventional low pressure bags. as the roof, sides, wings, bonnet and boot. FEATURES USED FOR • Lightweight and easily portable • Road traffic incidents • Provide a strong and steady lift to stabilize a vehicle and improve access to casualties INCLUDED • Exceptionally Stable • Valise (for up to two bags) • Strong and Durable • Repair kit • Non-slip top surface • Low maintenance costs • Large lift capacity and height • Small footprint for lift height • Controlled deflation capability 3 2 & 3 TIER LIFTING BAGS 2 TIER 3 TIER Product Code LB0067 LB0068 Materials Hypalon Coated Nylon Length (cm / inch) 55 / 21.7” 55 / 21.7” Width (cm / inch) 45 / 17.7” 45 / 17.7” Inflated Height (cm / inch) 40 / 15.7” 60 / 23.6” Deflated Height (cm / inch) 5 / 2” 5 / 2” Lift at Max Pressure (kg / lbs) 2150 / 4740 1780 / 3924 Air Requirements (ltr / ft3) 110 / 3.9 150 / 5.3 Packed Weight (kg) 10 / 22 15 / 32 Max Pressure (bar /psi) 1 / 14.5 1 / 14.5 SYSTEM SCHEMATIC d a c b 1 2 3 4 Low-Pressure Supply Either from BA cylinder & regulator or a compressor -

Draft Environmental Impact Statement
South Fork Flathead Watershed Westslope Cutthroat Trout Conservation Program Draft Environmental Impact Statement Responsible Agency: U.S. Department of Energy (DOE), Bonneville Power Administration (BPA) Cooperating Agencies: U.S. Department of Agriculture, Forest Service (FS) and State of Montana Fish, Wildlife, and Parks (MFWP) Department Title of Proposed Project: South Fork Flathead Watershed Westslope Cutthroat Trout Conservation Program State Involved: Montana Abstract: In cooperation with MFWP, BPA is proposing to implement a conservation program to preserve the genetic purity of the westslope cutthroat trout populations in the South Fork of the Flathead drainage. The South Fork Flathead Watershed Westslope Cutthroat Trout Conservation Program constitutes a portion of the Hungry Horse Mitigation Program. The purpose of the Hungry Horse Mitigation Program is to mitigate for the construction and operation of Hungry Horse Dam through restoring habitat, improving fish passage, protecting and recovering native fish populations, and reestablishing fish harvest opportunities. The target species for the Hungry Horse Mitigation Program are bull trout, westslope cutthroat trout, and mountain whitefish. The program is designed to preserve the genetically pure fluvial and adfluvial westslope cutthroat trout (Oncorhynchus clarki lewisi) populations in the South Fork drainage of the Flathead River. In order to accomplish the goals, MFWP is proposing to remove hybrid trout from identified lakes in the South Fork Flathead drainage on the Flathead National Forest and replace them with genetically pure native westslope cutthroat trout over the next 10-12 years. Some of these lakes occur within the Bob Marshall Wilderness and Jewel Basin Hiking Area. Currently, 21 lakes and their outflow streams with hybrid populations have been identified and are included in this proposal. -

Scientific Diving Techniques in Restricted Overhead Environments
doi:10.3723/ut.31.013 International Journal of the Society for Underwater Technology, Vol 31, No 1, pp 13–19, 2012 per Scientific diving techniques in restricted overhead environments *1,2 3 4 1 Giorgio Caramanna , Pirkko Kekäläinen , Jouni Leinikki and Mercedes Maroto-Valer Pa Technical 1Heriot-Watt University, Edinburgh, Scotland, UK 2Italian Association of Scientific Divers (AIOSS), Italy 3University of Helsinki, Finland 4Alleco Ltd, Finland Abstract applications (Auster et al., 1988; Auster, 1997; Scientific diving is an extremely useful tool for supporting Norcross and Mueter, 1999; Sarradin et al., 2002; research in environments with restricted access, where Bovio et al., 2006; Bowen et al., 2007), they cannot remotely operated or autonomous underwater vehicles can- always replace the presence of a scientific diver with not be used. However, these environments tend to be close regard to the quality and reliability of collected data. to the surface and require the application of advanced diving There are also environments, such as caves, under- techniques to ensure that the research is conducted within ice areas, springs or small lakes, in which access can acceptable safety parameters. The two main techniques be restricted and difficult to enter, and where use of discussed are under-ice and cave diving; for each environ- automated systems can be either complex or impos- ment the specific hazards are reviewed and methods for sible to achieve. Some of these restricted environ- mitigating the concomitant risks are detailed. It is concluded ments have related potential hazards for diving that that scientific diving operations in these environments can have to be correctly identified and addressed in be conducted to acceptable risk levels; however, risk man- agement strategies must outline precisely when and where order to guarantee that the underwater research is diving operations are to be prohibited or terminated. -

Jacks, Industrial Rollers, Air Casters, and Hydraulic Gantries
ASME B30.1-2015 (Revision of ASME B30.1-2009) Jacks, Industrial Rollers, Air Casters, and Hydraulic Gantries Safety Standard for Cableways, Cranes, Derricks, Hoists, Hooks, Jacks, and Slings AN AMERICAN NATIONAL STANDARD www.astaco.ir ASME B30.1-2015 (Revision of ASME B30.1-2009) Jacks, Industrial Rollers, Air Casters, and Hydraulic Gantries Safety Standard for Cableways, Cranes, Derricks, Hoists, Hooks, Jacks, and Slings AN AMERICAN NATIONAL STANDARD Two Park Avenue • New York, NY • 10016 USA www.astaco.ir Date of Issuance: May 29, 2015 The next edition of this Standard is scheduled for publication in 2020. This Standard will become effective 1 year after the Date of Issuance. ASME issues written replies to inquiries concerning interpretations of technical aspects of this Standard. Interpretations are published on the ASME Web site under the Committee Pages at http://cstools.asme.org/ as they are issued. Interpretations will also be included with each edition. Errata to codes and standards may be posted on the ASME Web site under the Committee Pages to provide corrections to incorrectly published items, or to correct typographical or grammatical errors in codes and standards. Such errata shall be used on the date posted. The Committee Pages can be found at http://cstools.asme.org/. There is an option available to automatically receive an e-mail notification when errata are posted to a particular code or standard. This option can be found on the appropriate Committee Page after selecting “Errata” in the “Publication Information” section. ASME is the registered trademark of The American Society of Mechanical Engineers. -

Www .Savatech.Com
www.savatech.com 1 CONTENTS HIGH-PRESSURE LIFTING BAGS 4 HIGH-PRESSURE FLAT-FORM LIFTING BAGS 6 HIGH-PRESSURE HOSE 7 MEDIUM-PRESSURE BAGS 8 MAXI-LIFT RECOVERY BAGS 9 TANK SEALING BAGS 10 PIPE SEALING BAGS 11 INFLATION ACCESSORIES 12 INFLATION ACCESSORIES 13 TANKS 14 SHELTERS - WILDERNESS RESCUE 15 Savatech Corp. 413 Oak Place, Bldg. 5J Port Orange, FL 32127 USA 888-436-9778 toll free 386-760-0706 International 386-760-8754 fax www.savatech.com [email protected] 2 APPLICATION High-pressure lifting bags For lifting the heaviest objects. Lift up to 74 tons per bag. Use these bags for sturdy objects. Use these bags for lifting, stabilizing or moving steel objects, motor vehicles, buildings, concrete or other sturdy structures. High-lift, medium-pressure lifting bags For lifting large objects or delicate structures such as aircraft, or light-weight aluminum structures. Low-pressure, high-lift, lifting bags exerte a low concentrated lifting force over a wide area. High-pressure Flat-Form lifting bags HighH lift and high capacity. The newest technology in the high-pressure liftingl bag industry. Use these bags when extra stability is desired. Higher lift can be achieved by stacking more bags. Maxi-lift recovery lifting bags For lifting the largest objects. Excellent for delicate structures such as soft-side tractor-trailers, aircraft, or light-weight aluminum structures. Low-pressure lifting bags exert low concentrated force over a wider area. Leak sealing bags For temporary leak sealing. Use for tanks, pipes, containers of all shapes and sizes. Can be used for the most hazardous liquids, fuels, water,w oils, etc. -

Severe Hypoxemia During Apnea in Humans: Influence of Cardiovascular Responses
From the Department of Physiology and Pharmacology Section of Environmental Physiology Karolinska Institutet Stockholm, Sweden Severe hypoxemia during apnea in humans: influence of cardiovascular responses by Peter Lindholm Stockholm 2002 ABSTRACT When a diving human holds his or her breath, the heart beat slows and the blood vessels constrict in large portions of the body. In diving mammals such as seals, similar responses effectively conserve oxygen for the brain, enabling them to dive very deep and to stay underwater for a long time. The principal aim of this thesis was to study the biological significance of bradycardia (a slowing of the heart rate) and vasoconstriction during apnea in exercising humans. The question was whether these cardiovascular mechanisms also in humans would temporarily “conserve” oxygen (i.e. temporarily reduce O2 uptake in muscles etc.) and thus protect the brain from a level of hypoxia that would otherwise cause unconsciousness in a breath-hold diver. A second aim was to determine the inter-individual variation among the human subjects in terms of the degree of bradycardia during apnea, in order to explore whether the intensity of these cardiovascular responses would make some individuals more fit to survive underwater swimming and apnea than others. Our results indeed showed inter-individual differences such that a high degree of bradycardia during apnea correlated significantly with higher levels (= better conservation) of arterial oxygen saturation. Thus, the subjects with the lowest heart rates during exercise and apnea had the best preserved saturation measured with earlobe pulse oximetry. Also, we found that the cardiovascular responses to apnea in exercising humans clearly delay the development of hypoxemia by reducing the rate of uptake from the main oxygen store, i.e. -

Underwater Air Lift Bags
AB AB The International Marine Contractors Association Underwater Air Lift Bags The International Marine Contractors Association IMCA D 016 Rev. 3 www.imca-int.com June 2007 www.imca-int.com AB The International Marine Contractors Association (IMCA) is the international trade association representing offshore, marine and underwater engineering companies. IMCA promotes improvements in quality, health, safety, environmental and technical standards through the publication of information notes, codes of practice and by other appropriate means. Members are self-regulating through the adoption of IMCA guidelines as appropriate. They commit to act as responsible members by following relevant guidelines and being willing to be audited against compliance with them by their clients. There are two core activities that relate to all members: Safety, Environment & Legislation Training, Certification & Personnel Competence The Association is organised through four distinct divisions, each covering a specific area of members’ interests: Diving, Marine, Offshore Survey, Remote Systems & ROV. There are also four regional sections which facilitate work on issues affecting members in their local geographic area – Americas Deepwater, Asia-Pacific, Europe & Africa and Middle East & India. IMCA D 016 Rev. 3 This update of earlier IMCA and AODC guidance documents was prepared by IMCA, under the direction of its Diving Division Management Committee. This document specifically supersedes IMCA D 016 Rev. 2, dated May 2003, which is now withdrawn. www.imca-int.com/diving The information contained herein is given for guidance only and endeavours to reflect best industry practice. For the avoidance of doubt no legal liability shall attach to any guidance and/or recommendation and/or statement herein contained.