Dive Theory Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Surfactants on the Toxicitiy of Glyphosate, with Specific Reference to RODEO
SERA TR 97-206-1b Effects of Surfactants on the Toxicitiy of Glyphosate, with Specific Reference to RODEO Submitted to: Leslie Rubin, COTR Animal and Plant Health Inspection Service (APHIS) Biotechnology, Biologics and Environmental Protection Environmental Analysis and Documentation United States Department of Agriculture Suite 5A44, Unit 149 4700 River Road Riverdale, MD 20737 Task No. 5 USDA Contract No. 53-3187-5-12 USDA Order No. 43-3187-7-0028 Prepared by: Gary L. Diamond Syracuse Research Corporation Merrill Lane Syracuse, NY 13210 and Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Telephone: (315) 637-9560 Fax: (315) 637-0445 CompuServe: 71151,64 Internet: [email protected] February 6, 1997 TABLE OF CONTENTS EXECUTIVE SUMMARY ......................................... iii 1. INTRODUCTION .............................................1 2. CONSTITUENTS OF SURFACTANT FORMULATIONS USED WITH RODE0 ....4 2.1. AGRI-DEX ..........................................4 2.2. LI-700 .............................................4 2.3. R-11 ...............................................6 2.4. LATRON AG-98 ......................................6 2.5. LATRON AG-98 AG ....................................7 3. INFORMATION ON EFFECTS OF SURFACTANT FORMULATIONS ON THE TOXICITY OF RODEO ................................8 3.1. HUMAN HEALTH ......................................8 3.1.1. LI 700 ........................................8 3.1.2. Phosphatidylcholine ................................8 -

Standard Operating Procedures for Scientific Diving
Standard Operating Procedures for Scientific Diving The University of Texas at Austin Marine Science Institute 750 Channel View Drive, Port Aransas Texas 78373 Amended January 9, 2020 1 This standard operating procedure is derived in large part from the American Academy of Underwater Sciences standard for scientific diving, published in March of 2019. FOREWORD “Since 1951 the scientific diving community has endeavored to promote safe, effective diving through self-imposed diver training and education programs. Over the years, manuals for diving safety have been circulated between organizations, revised and modified for local implementation, and have resulted in an enviable safety record. This document represents the minimal safety standards for scientific diving at the present day. As diving science progresses so must this standard, and it is the responsibility of every member of the Academy to see that it always reflects state of the art, safe diving practice.” American Academy of Underwater Sciences ACKNOWLEDGEMENTS The Academy thanks the numerous dedicated individual and organizational members for their contributions and editorial comments in the production of these standards. Revision History Approved by AAUS BOD December 2018 Available at www.aaus.org/About/Diving Standards 2 Table of Contents Volume 1 ..................................................................................................................................................... 6 Section 1.00 GENERAL POLICY ........................................................................................................................ -

Full Paper/Talk Deep Stops and Shallow Stops – Fact and Fancy
Full Paper/Talk Deep Stops and Shallow Stops – Fact and Fancy B.R. Wienke1 and T.R. O’Leary2 1 C&C Dive Team Ldr, Program Manager Computational Physics, LANL, MS D490, Los Alamos, NM 87545 [email protected] 2 Director, NAUI Technical Diving Operations, 33256 State Rd 100, South Padre Island, TX, 78597 Abstract The question of deep stops and shallow stops is interesting and fraught with controversy in diving circles and operations, training, exploration and scientific endeavors. Plus frought with some misunderstanding which is understandable as the issues are complex. We therefore attempt a short history of deep and shallow stops, physical aspects, staging differences, diving tests, models with data correlations and data banks with user statistics and DCS outcomes as diver amplification. Pros and cons of both deep stop and shallow stop staging are presented. Misfacts are righted when appropriate. Chamber, wet and Doppler tests are contrasted. A compendium of Training Agency Standards regarding deep and shallow stops is included. Dive software is also detailed. Some commercial diving operations are discussed. A short tabulation of dive computer and software algorithms is given. From diving data, tests, DCS outcomes and field usage, we conclude that both deep stops and shallow stops are safely employed in recreational and technical diving. That is a good thing but choose your deco wisely and know why. Keywords: computational models, decompression staging, profile data, risk, statistical correlations, tests Acronyms and Nomenclature ANDI: Association of Nitrox Diving Instructors. BM: bubble phase model dividing the body into tissue compartments with halftimes that are coupled to inert gas diffusion across bubble film surfaces of exponential size distribution constrained in cumulative growth by a volume limit point. -

Pulmonary Surfactant: the Key to the Evolution of Air Breathing Christopher B
Pulmonary Surfactant: The Key to the Evolution of Air Breathing Christopher B. Daniels and Sandra Orgeig Department of Environmental Biology, University of Adelaide, Adelaide, South Australia 5005, Australia Pulmonary surfactant controls the surface tension at the air-liquid interface within the lung. This sys- tem had a single evolutionary origin that predates the evolution of the vertebrates and lungs. The lipid composition of surfactant has been subjected to evolutionary selection pressures, partic- ularly temperature, throughout the evolution of the vertebrates. ungs have evolved independently on several occasions pendent units, do not necessarily stretch upon inflation but Lover the past 300 million years in association with the radi- unpleat or unfold in a complex manner. Moreover, the many ation and diversification of the vertebrates, such that all major fluid-filled corners and crevices in the alveoli open and close vertebrate groups have members with lungs. However, lungs as the lung inflates and deflates. differ considerably in structure, embryological origin, and Surfactant in nonmammals exhibits an antiadhesive func- function between vertebrate groups. The bronchoalveolar lung tion, lining the interface between apposed epithelial surfaces of mammals is a branching “tree” of tubes leading to millions within regions of a collapsed lung. As the two apposing sur- of tiny respiratory exchange units, termed alveoli. In humans faces peel apart, the lipids rise to the surface of the hypophase there are ~25 branches and 300 million alveoli. This structure fluid at the expanding gas-liquid interface and lower the sur- allows for the generation of an enormous respiratory surface face tension of this fluid, thereby decreasing the work required area (up to 70 m2 in adult humans). -

Ear Clearing for Non-Divers
Scuba School Ltd – Ear Clearing for non-divers Ear clearing or clearing the ears or equalization is any of various maneuvers to equalize the pressure in the middle ear with the outside pressure, by letting air enter along the Eustachian tubes, as this does not always happen automatically when the pressure in the middle ear is lower than the outside pressure. This need can arise in scuba diving, freediving/spearfishing, skydiving, fast descent in an aircraft, fast descent in a mine cage, and being put into pressure in a caisson or similar pressure-bearing structure, or sometimes even simply travelling at fast speeds in an automobile. People who do intense weight lifting, like squats, may experience sudden conductive hearing loss due to air pressure building up inside the ear. They are advised to engage in an ear clearing method to relieve pressure, or pain if any. Methods The ears can be cleared by various methods,[5] some of which pose a distinct risk of barotrauma including perforation of the eardrum: Yawning which helps to open the eustachian tubes; Swallowing which helps to open the eustachian tubes; The "Frenzel maneuver": Using the rear part of the tongue and throat muscles, close the nostrils, and close the back of the throat as if straining to lift a weight. Then make the sound of the letter "K." This pushes the back of the tongue upward, compressing air into the openings of the eustachian tubes. "Politzerization": a medical procedure that involves inflating the middle ear by blowing air up the nose during the act of swallowing; The "Toynbee maneuver": pinching the nose and swallowing. -

Henry's Law Constants and Micellar Partitioning of Volatile Organic
38 J. Chem. Eng. Data 2000, 45, 38-47 Henry’s Law Constants and Micellar Partitioning of Volatile Organic Compounds in Surfactant Solutions Leland M. Vane* and Eugene L. Giroux United States Environmental Protection Agency, National Risk Management Research Laboratory, 26 West Martin Luther King Drive, Cincinnati, Ohio 45268 Partitioning of volatile organic compounds (VOCs) into surfactant micelles affects the apparent vapor- liquid equilibrium of VOCs in surfactant solutions. This partitioning will complicate removal of VOCs from surfactant solutions by standard separation processes. Headspace experiments were performed to quantify the effect of four anionic surfactants and one nonionic surfactant on the Henry’s law constants of 1,1,1-trichloroethane, trichloroethylene, toluene, and tetrachloroethylene at temperatures ranging from 30 to 60 °C. Although the Henry’s law constant increased markedly with temperature for all solutions, the amount of VOC in micelles relative to that in the extramicellar region was comparatively insensitive to temperature. The effect of adding sodium chloride and isopropyl alcohol as cosolutes also was evaluated. Significant partitioning of VOCs into micelles was observed, with the micellar partitioning coefficient (tendency to partition from water into micelle) increasing according to the following series: trichloroethane < trichloroethylene < toluene < tetrachloroethylene. The addition of surfactant was capable of reversing the normal sequence observed in Henry’s law constants for these four VOCs. Introduction have long taken advantage of the high Hc values for these compounds. In the engineering field, the most common Vast quantities of organic solvents have been disposed method of dealing with chlorinated solvents dissolved in of in a manner which impacts human health and the groundwater is “pump & treat”swithdrawal of ground- environment. -

Deadly Acute Decompression Sickness in Risso's Dolphins
www.nature.com/scientificreports OPEN Deadly acute Decompression Sickness in Risso’s dolphins A. Fernández, E. Sierra, J. Díaz-Delgado, S. Sacchini , Y. Sánchez-Paz, C. Suárez-Santana, M. Arregui, M. Arbelo & Y. Bernaldo de Quirós Received: 19 April 2017 Diving air-breathing vertebrates have long been considered protected against decompression sickness Accepted: 5 October 2017 (DCS) through anatomical, physiological, and behavioural adaptations. However, an acute systemic gas Published: xx xx xxxx and fat embolic syndrome similar to DCS in human divers was described in beaked whales that stranded in temporal and spatial association with military exercises involving high-powered sonar. More recently, DCS has been diagnosed in bycaught sea turtles. Both cases were linked to human activities. Two Risso’s dolphin (Grampus griseus) out of 493 necropsied cetaceans stranded in the Canary Islands in a 16-year period (2000–2015), had a severe acute decompression sickness supported by pathological fndings and gas analysis. Deadly systemic, infammatory, infectious, or neoplastic diseases, ship collision, military sonar, fsheries interaction or other type of lethal inducing associated trauma were ruled out. Struggling with a squid during hunting is discussed as the most likely cause of DCS. Pathologies related to efects of changes in pressure are well known among human divers. Decompression sick- ness (DCS) is a syndrome related to the formation of gas bubbles in blood and/or tissues when the sum of the dissolved gas tensions exceeds the local absolute pressure. Gas bubbles may have biochemical efects and disrupt the tissues or occlude the vessels with clinical and pathological signs and, in certain cases, death1. -

Biomechanics of Safe Ascents Workshop
PROCEEDINGS OF BIOMECHANICS OF SAFE ASCENTS WORKSHOP — 10 ft E 30 ft TIME AMERICAN ACADEMY OF UNDERWATER SCIENCES September 25 - 27, 1989 Woods Hole, Massachusetts Proceedings of the AAUS Biomechanics of Safe Ascents Workshop Michael A. Lang and Glen H. Egstrom, (Editors) Copyright © 1990 by AMERICAN ACADEMY OF UNDERWATER SCIENCES 947 Newhall Street Costa Mesa, CA 92627 All Rights Reserved No part of this book may be reproduced in any form by photostat, microfilm, or any other means, without written permission from the publishers Copies of these Proceedings can be purchased from AAUS at the above address This workshop was sponsored in part by the National Oceanic and Atmospheric Administration (NOAA), Department of Commerce, under grant number 40AANR902932, through the Office of Undersea Research, and in part by the Diving Equipment Manufacturers Association (DEMA), and in part by the American Academy of Underwater Sciences (AAUS). The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding the copyright notation that appears above. Opinions presented at the Workshop and in the Proceedings are those of the contributors, and do not necessarily reflect those of the American Academy of Underwater Sciences PROCEEDINGS OF THE AMERICAN ACADEMY OF UNDERWATER SCIENCES BIOMECHANICS OF SAFE ASCENTS WORKSHOP WHOI/MBL Woods Hole, Massachusetts September 25 - 27, 1989 MICHAEL A. LANG GLEN H. EGSTROM Editors American Academy of Underwater Sciences 947 Newhall Street, Costa Mesa, California 92627 U.S.A. An American Academy of Underwater Sciences Diving Safety Publication AAUSDSP-BSA-01-90 CONTENTS Preface i About AAUS ii Executive Summary iii Acknowledgments v Session 1: Introductory Session Welcoming address - Michael A. -
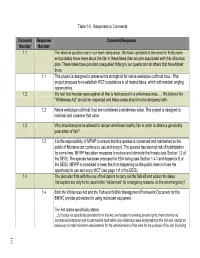
Final EIS Responses to Comments 1-40
Table 1-2. Responses to Comments Comment Response Comment/Response Number Number 1.1 The lakes in question are in our main camp area. We have operated in this area for thirty years and probably know more about the fish in these lakes than anyone associated with this ridiculous plan. These lakes have provided unequalled fishing to our guests and all others that have fished them. 1.1 This project is designed to preserve this stronghold for native westslope cutthroat trout. This project proposes to re-establish WCT populations in all treated lakes, which will maintain angling opportunities. 1.2 We feel that his plan goes against all that is held sacred in a wilderness area. … We believe the "Wilderness Act" should be respected and these areas should not be tampered with. 1.2 Native westslope cutthroat trout are considered a wilderness value. This project is designed to maintain and conserve that value. 1.3 Why should anyone be allowed to tamper with these healthy fish in order to obtain a genetically pure strain of fish? 1.3 It is the responsibility of MFWP to ensure that this species is conserved and maintained so the public of Montana can continue to use and enjoy it. The species has been at risk of hybridization for some time. MFWP has taken measures to reduce and eliminate the threats (see Section 1.2 of the DEIS). The species has been proposed for ESA listing (see Section 1.4.1 and Appendix B of the DEIS). MFWP is mandated to keep this from happening so the public does not lose the opportunity to use and enjoy WCT (see page 1-8 of the DEIS). -

Training Objectives for a Diving Medical Physician
The Diving Medical Advisory Committee Training Objectives for a Diving Medicine Physician This guidance includes all the training objectives agreed by the Diving Medical Advisory Committee, the European Diving Technology Committee and the European Committee for Hyperbaric Medicine in 2011. Rev 1 - 2013 INTRODUCTION The purpose of this document is to define more closely the training objectives in diving physiology and medicine that need to be met by doctors already fully accredited or board-certified in a clinical speciality to national standards. It is based on topic headings that were originally prepared for a working group of European Diving Technology Committee (EDTC) and the European Committee of Hyperbaric Medicine (ECHM) as a guide for diving medicine some 20 years ago by J.Desola (Spain), T.Nome (Norway) & D.H.Elliott (U.K.). The training now required for medical examiners of working divers and for specialist diving medicine physicians was based on a EDTC/ECHM standard 1999 and subsequently has been enhanced by the Diving Medical Advisory Committee (DMAC), revised and agreed in principle by DMAC, EDTC and ECHM in 2010 and then ratified by EDTC and ECHM in 2011. The requirements now relate to an assessment of competence, the need for some training in occupational medicine, the need for maintenance of those skills by individual ‘refresher training’. Formal recognition of all this includes the need to involve a national authority for medical education. These objectives have been applied internationally to doctors who provide medical support to working divers. (Most recreational instructors and dive guides are, by their employment, working divers and so the guidance includes the relevant aspects of recreational diving. -

Quality Rental Tools and Equipment– Well Serviced, Priced Right
RENTAL CATALOG QUALITY RENTAL TOOLS AND EQUIPMENT– WELL SERVICED, PRICED RIGHT. RENTAL INDEX CORE DRILLS SAFETY AIR COMPRESSORS Core Drills . 1 Confined Space Tripod . 13 Air Compressors - Tow Behind . 24 Accessories . 1 Davit System . 13 Air Compressors - Electric . 24 Bits . 2 Fall Protection Carts . 13 Retractable Lifelines . 13 BREAKERS ELECTRICAL Horizontal Lifelines . 13 Air (30lb, 60lb and 90lb) . 25 Cable Cutters . 3 Gas Monitors . 13 Electric . 25 Cable Crimpers . 3 Monitor Pumps . 13 Air Hose . 25 Cable Pullers . 3 Cable Sheaves . 3 BLOWERS BAKER SCAFFOLD Pull Monitor . 3 Blowers . 14 Baker Scaffold . 25 Cable Feeder . 5 Reel Stands . 5 MATERIAL LIFTS AIR TOOLS Reel Stand Spindles . 5 Material Lifts . 14 Rivet Buster . 26 Rod and Strut Cutters . 5 Roustabouts . 14 Rivet Buster Bits . .26 Conduit Benders . 5-6 A/C Lift and Mover . 14 Scalers . 26 Conduit Cart . 6 Gantry . .15 Chippers . 26 Knock Out Sets . 6 Bits for Air Chippers . 26 Cable Stripper . 6 HYDRAULIC CYLINDERS Impacts . 26 Hydraulic Cylinders/Bags . 15-16 Air Hose . 26 RADIOS Hydraulic Pumps and Hoses . 16 Saws . 28 Radios . 6 TORQUE TOOLS COMPACTION PIPING TOOLS Hand Torque Wrenches . 16 Plate Compactor . .28 Threaders . 7 Cordless Torque Wrenches . 18 Jumping Jack Tampers . 28 Threader Accessories . 7 Hydraulic Torque Wrenches . 18 Roll Groovers . 7 Punches . 18 GENERATORS AND PUMPS Groover Accessories . 9 Generators . 28 See Snake Camera . 9 CHAIN HOISTS Gas Pumps . 29 Locators . 9 Hand Chainfall Hoist . 18-19 Electric Pumps . 29 Pipe Freeze Kits . 9 Hand Lever Hoist (Come-A-Long) . 19 Pipe Thawers . 9 Electric Chainfalls . 20-21 WELDING T-Drills . 9 Air Hoist . -

A Primer for Technical Diving Decompression Theory
SCUBA AA PPRRIIMMEERR FFOORR TECH TTEECCHHNNIICCAALL DDIIVVIINNGG DDEECCOOMMPPRREESSSSIIOONN PHILIPPINES TTHHEEOORRYY 1 | P a g e ©Andy Davis 2015 www.scubatechphilippines.com Sidemount, Technical & Wreck Guide | Andy Davis First Published 2016 All documents compiled in this publication are open-source and freely available on the internet. Copyright Is applicable to the named authors stated within the document. Cover and logo images are copyright to ScubaTechPhilippines/Andy Davis. Not for resale. This publication is not intended to be used as a substitute for appropriate dive training. Diving is a dangerous sport and proper training should only be conducted under the safe supervision of an appropriate, active, diving instructor until you are fully qualified, and then, only in conditions and circumstances which are as good or better than the conditions in which you were trained. Technical scuba diving should be taught by a specialized instructor with training credentials and experience at that level of diving. Careful risk assessment, continuing education and skill practice may reduce your likelihood of an accident, but are in no means a guarantee of complete safety. This publication assumes a basic understanding of diving skills and knowledge. It should be used to complement the undertaking of prerequisite training on the route to enrolling upon technical diving training. 2 | P a g e ©Andy Davis 2015 www.scubatechphilippines.com This primer on decompression theory is designed as a supplement to your technical diving training. Becoming familiar with the concepts and terms outlined in this document will enable you to get the most out of your theory training with me; and subsequently enjoy safer, more refined dive planning and management in your technical diving activities.