Severe Hypoxemia During Apnea in Humans: Influence of Cardiovascular Responses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introducing Finswimming
LIFESTYLE by Jasmin Wong PART-TIME: Introducing MERMAIDS Finswimming Lifestyle t is not hard to spot Lee Ho-kui as Hong Kong team athletes for open enjoy the satisfaction of achieving great he heads towards the Lei Cheng finswimming competitions. speed and the experience of snorkelling. IUk Swimming Pool to attend a finswimming training session. It is not “To put it simply, finswimming For instance, the fastest record for that he is wearing a conspicuous outfit is a combination of swimming and swimming 50-metres free-style is 21 but rather it is the large fin he is carrying snorkelling, a recreation and a seconds. A finswimmer can knock five that is drawing the gaze of various competitive sport,” explains Lee. seconds off that time. Lee says it is the passers-bys. It is in fact a monofin, a fin- Finswimmers use a monofin for attraction of extra speed that appeals to shaped piece of equipment used for the propulsion and a snorkel for breathing, so many seasoned swimmers. sport of finswimming. allowing them to go much faster than conventional swimmers. The Finswimming competitions are The 34-year-old advanced finswimming technique of finswimming is very divided into different categories, instructor, who has been working for different to ordinary swimming. All namely Surface, Apnea, Immersion the Hong Kong Underwater Association propulsion is done with a swim fin, and Bifin. With each event having (HKUA) for seven years, describes using the legs and lower body in an a completely different set of his interest in finswimming as “an undulating, up-and-down movement. -

Monofins for Freediving
Monofins for Freediving We have been intermittently following the debate concerning the use of the monofin in freediving and would like to share some of our findings. Two years ago we put together the first experimental monofin/freedive clinic where we assembled some unique elements. We put together the leading trainers in monofin swimming, namely the Russian coaches from Tomsk university, who train both the Russian national team and their chief rivals, the Chinese, the leading specialist monofin manufacturer belonging to the same school and a group of freedivers which represented the best cross-section, from the very top of freediving competition to the very novice. This same group also represented advanced freedivers who already had experience with the monofin, advanced freedivers who had never used a monofin and a novice freediver with no experience of the monofin. Although the number of freedivers involved was small we feel that with a larger group the conclusions would have been much the same. The objectives were to find (i) What style and why? (ii) What rhythm and amplitude of movement? (iii) What kind of monofin and what stiffness of blade and if this was individual what the relevant criteria for monofin choice should be? (iv) What compromises and adaptations had to be made to suit the specific needs of the freediver? (v) What was the best training method for the monofin freediver. What style and why? We had heard a lot of talk concerning adaptations of the ‘classic ’style that freedivers should adopt. I know from personal acquaintance that some of the people recommending various adaptations were not capable of demonstrating a good classic style hence their recommendations were from lack of ability in the monofin and hence lack of choice through limited ability. -

APNEA TRAINING and PHYSICAL CHARACTERISTICS: ENHANCEMENT of the DIVE RESPONSE, APNEIC TIME, and RECOVERY by Nathanael Stanford A
APNEA TRAINING AND PHYSICAL CHARACTERISTICS: ENHANCEMENT OF THE DIVE RESPONSE, APNEIC TIME, AND RECOVERY by Nathanael Stanford A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Kinesiology Boise State University May 2019 © 2019 Nathanael Stanford ALL RIGHTS RESERVED BOISE STATE UNIVERSITY GRADUATE COLLEGE DEFENSE COMMITTEE AND FINAL READING APPROVALS of the thesis submitted by Nathanael Stanford Thesis Title: Apnea Training and Physical Characteristics: Enhancement of The Dive Response, Apneic Time, and Recovery Date of Final Oral Examination: 08 March 2019 The following individuals read and discussed the thesis submitted by student Nathanael Stanford, and they evaluated his presentation and response to questions during the final oral examination. They found that the student passed the final oral examination. Shawn R. Simonson, Ed.D. Chair, Supervisory Committee Timothy R. Kempf, Ph.D. Member, Supervisory Committee Jeffrey M. Anderson, MA Member, Supervisory Committee The final reading approval of the thesis was granted by Shawn R. Simonson, Ed.D., Chair of the Supervisory Committee. The thesis was approved by the Graduate College. ACKNOWLEDGEMENTS I would first like to thank my thesis advisor, Shawn Simonson for his continual assistance and guidance throughout this master thesis. He fostered an environment that encouraged me to think critically about the scientific process. The mentorship he offered directed me on a path of independent thinking and learning. I would like to thank my research technician, Sarah Bennett for the hundreds of hours she assisted me during data collection. This thesis would not have been possible without her help. To my committee member Tim Kempf, I want to express my gratitude for his assistance with study design and the scientific writing process. -

Dive Theory Guide
DIVE THEORY STUDY GUIDE by Rod Abbotson CD69259 © 2010 Dive Aqaba Guidelines for studying: Study each area in order as the theory from one subject is used to build upon the theory in the next subject. When you have completed a subject, take tests and exams in that subject to make sure you understand everything before moving on. If you try to jump around or don’t completely understand something; this can lead to gaps in your knowledge. You need to apply the knowledge in earlier sections to understand the concepts in later sections... If you study this way you will retain all of the information and you will have no problems with any PADI dive theory exams you may take in the future. Before completing the section on decompression theory and the RDP make sure you are thoroughly familiar with the RDP, both Wheel and table versions. Use the appropriate instructions for use guides which come with the product. Contents Section One PHYSICS ………………………………………………page 2 Section Two PHYSIOLOGY………………………………………….page 11 Section Three DECOMPRESSION THEORY & THE RDP….……..page 21 Section Four EQUIPMENT……………………………………………page 27 Section Five SKILLS & ENVIRONMENT…………………………...page 36 PHYSICS SECTION ONE Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through the glass of the mask and the air space, the difference in speed causes the light rays to bend; this is called refraction. To the diver wearing a normal diving mask objects appear to be larger and closer than they actually are. -
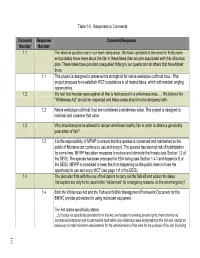
Final EIS Responses to Comments 1-40
Table 1-2. Responses to Comments Comment Response Comment/Response Number Number 1.1 The lakes in question are in our main camp area. We have operated in this area for thirty years and probably know more about the fish in these lakes than anyone associated with this ridiculous plan. These lakes have provided unequalled fishing to our guests and all others that have fished them. 1.1 This project is designed to preserve this stronghold for native westslope cutthroat trout. This project proposes to re-establish WCT populations in all treated lakes, which will maintain angling opportunities. 1.2 We feel that his plan goes against all that is held sacred in a wilderness area. … We believe the "Wilderness Act" should be respected and these areas should not be tampered with. 1.2 Native westslope cutthroat trout are considered a wilderness value. This project is designed to maintain and conserve that value. 1.3 Why should anyone be allowed to tamper with these healthy fish in order to obtain a genetically pure strain of fish? 1.3 It is the responsibility of MFWP to ensure that this species is conserved and maintained so the public of Montana can continue to use and enjoy it. The species has been at risk of hybridization for some time. MFWP has taken measures to reduce and eliminate the threats (see Section 1.2 of the DEIS). The species has been proposed for ESA listing (see Section 1.4.1 and Appendix B of the DEIS). MFWP is mandated to keep this from happening so the public does not lose the opportunity to use and enjoy WCT (see page 1-8 of the DEIS). -

Using Underwater Pulse Oximetry in Freediving to Extreme Depths to Study Risk of Hypoxic Blackout and Diving Response Phases
ORIGINAL RESEARCH published: 01 April 2021 doi: 10.3389/fphys.2021.651128 Using Underwater Pulse Oximetry in Freediving to Extreme Depths to Study Risk of Hypoxic Blackout and Diving Response Phases Eric Mulder 1* and Erika Schagatay 1,2 1 Environmental Physiology Group, Department of Health Sciences, Mid Sweden University, Östersund, Sweden, 2 Swedish Winter Sports Research Centre, Mid Sweden University, Östersund, Sweden Deep freediving exposes humans to hypoxia and dramatic changes in pressure. The effect of depth on gas exchange may enhance risk of hypoxic blackout (BO) during the last part of the ascent. Our aim was to investigate arterial oxygen saturation (SpO2) and heart rate Edited by: (HR) in shallow and deep freedives, central variables, which have rarely been studied Costantino Balestra, underwater in deep freediving. Four male elite competitive freedivers volunteered to wear Haute École Bruxelles-Brabant (HE2B), Belgium a newly developed underwater pulse oximeter for continuous monitoring of SpO2 and HR Reviewed by: during self-initiated training in the sea. Two probes were placed on the temples, connected Neal William Pollock, to a recording unit on the back of the freediver. Divers performed one “shallow” and one Laval University, Canada Kay Tetzlaff, “deep” constant weight dive with fins. Plethysmograms were recorded at 30 Hz, and University Hospital of Tübingen, SpO2 and HR were extracted. Mean ± SD depth of shallow dives was 19 ± 3 m, and Germany 73 ± 12 m for deep dives. Duration was 82 ± 36 s in shallow and 150 ± 27 s in deep Claus-Martin Muth, Universitaetsklinikum Ulm, Germany dives. All divers desaturated more during deeper dives (nadir 55 ± 10%) compared to *Correspondence: shallow dives (nadir 80 ± 22%) with a lowest SpO2 of 44% in one deep dive. -

The Effect of Breath-Hold Diving on Selected Adaptive Mechanisms in the Circulatory-Respiratory System in Simulated Static and Dynamic Apnoea
Baltic Journal of Health and Physical Activity 2019; 11(1): 7-17 Journal of Gdansk University of Physical Education and Sport ORIGINAL e-ISSN 2080-9999 doi: 10.29359/BJHPA.11.1.01 The effect of breath-hold diving on selected adaptive mechanisms in the circulatory-respiratory system in simulated static and dynamic apnoea 1 ABDF 1 D 2 C Authors’ Contribution: Magdalena Solich-Talanda , Rafał Mikołajczyk , Robert Roczniok , Aleksandra A Study Design Żebrowska1 ADEF B Data Collection C Statistical Analysis 1 D Data Interpretation Department of Physiological and Medical Sciences, E Manuscript Preparation The Jerzy Kukuczka Academy of Physical Education in Katowice, Poland F Literature Search G Funds Collection 2 Department of Sports Theory, The Jerzy Kukuczka Academy of Physical Education in Katowice, Poland abstract Background: Current research results indicate high adaptation of an organism to long-term apnoea. Breathing techniques allow increasing the volume of the inhaled air and thus prolong the breath-hold time at rest and during physical effort. The purpose of this study was to assess the impact of breath-hold on adaptations of the respiratory and circulatory systems and cardiopulmonary-respiratory reactions at rest and during physical effort in persons practising freediving. Material and methods: The study involved 17 athletes practising breath-hold diving, at the mean age of 38.4 ±8.4 years. Spirometry tests to evaluate static and dynamic lung indicators were conducted. The heart rate (HR), oxygen saturation (SpO2), and the apnoea time in three breath-hold trials were measured: static dry STA-D, static with face immersed in water STA-I and dynamic (DYN-D). -

Fort Riley Military Munitions Response Program Camp Forsyth Landfill Area 2 Munitions Response Site Operable Unit 09, FTRI-003-R-01 Geary County, Kansas U.S
Final Record of Decision June 2020 Fort Riley Military Munitions Response Program Camp Forsyth Landfill Area 2 Munitions Response Site Operable Unit 09, FTRI-003-R-01 Geary County, Kansas U.S. Army Corps of Engineers Omaha District FORT RILEY Final Contract No.: W912DQ-17-D-3023 Delivery Order No.: W9128F-17-F-0233 Record of Decision MILITARY MUNITIONS RESPONSE PROGRAM FORT RILEY CAMP FORSYTH LANDFILL AREA 2 MUNITIONS RESPONSE SITE OPERABLE UNIT 09, FTRI-003-R-01 GEARY COUNTY, KANSAS Prepared for and Prepared by U.S. ARMY CORPS OF ENGINEERS Omaha District June 2020 Revision 01 Record of Decision Camp Forsyth Landfill Area 2 MRS, Fort Riley, Kansas Table of Contents 1.0 DECLARATION .......................................................................................................... 1-1 1.1 Site Name and Location.................................................................................................... 1-1 1.2 Statement of Basis and Purpose...................................................................................... 1-1 1.3 Assessment of Site ............................................................................................................ 1-1 1.4 Description of Selected Remedy ...................................................................................... 1-1 1.5 Statutory Determinations .................................................................................................. 1-1 1.5.1 Part 1: Statutory Requirements ................................................................................ -

Quality Rental Tools and Equipment– Well Serviced, Priced Right
RENTAL CATALOG QUALITY RENTAL TOOLS AND EQUIPMENT– WELL SERVICED, PRICED RIGHT. RENTAL INDEX CORE DRILLS SAFETY AIR COMPRESSORS Core Drills . 1 Confined Space Tripod . 13 Air Compressors - Tow Behind . 24 Accessories . 1 Davit System . 13 Air Compressors - Electric . 24 Bits . 2 Fall Protection Carts . 13 Retractable Lifelines . 13 BREAKERS ELECTRICAL Horizontal Lifelines . 13 Air (30lb, 60lb and 90lb) . 25 Cable Cutters . 3 Gas Monitors . 13 Electric . 25 Cable Crimpers . 3 Monitor Pumps . 13 Air Hose . 25 Cable Pullers . 3 Cable Sheaves . 3 BLOWERS BAKER SCAFFOLD Pull Monitor . 3 Blowers . 14 Baker Scaffold . 25 Cable Feeder . 5 Reel Stands . 5 MATERIAL LIFTS AIR TOOLS Reel Stand Spindles . 5 Material Lifts . 14 Rivet Buster . 26 Rod and Strut Cutters . 5 Roustabouts . 14 Rivet Buster Bits . .26 Conduit Benders . 5-6 A/C Lift and Mover . 14 Scalers . 26 Conduit Cart . 6 Gantry . .15 Chippers . 26 Knock Out Sets . 6 Bits for Air Chippers . 26 Cable Stripper . 6 HYDRAULIC CYLINDERS Impacts . 26 Hydraulic Cylinders/Bags . 15-16 Air Hose . 26 RADIOS Hydraulic Pumps and Hoses . 16 Saws . 28 Radios . 6 TORQUE TOOLS COMPACTION PIPING TOOLS Hand Torque Wrenches . 16 Plate Compactor . .28 Threaders . 7 Cordless Torque Wrenches . 18 Jumping Jack Tampers . 28 Threader Accessories . 7 Hydraulic Torque Wrenches . 18 Roll Groovers . 7 Punches . 18 GENERATORS AND PUMPS Groover Accessories . 9 Generators . 28 See Snake Camera . 9 CHAIN HOISTS Gas Pumps . 29 Locators . 9 Hand Chainfall Hoist . 18-19 Electric Pumps . 29 Pipe Freeze Kits . 9 Hand Lever Hoist (Come-A-Long) . 19 Pipe Thawers . 9 Electric Chainfalls . 20-21 WELDING T-Drills . 9 Air Hoist . -

Cardiac Hypoxic Resistance and Decreasing Lactate During Maximum
www.nature.com/scientificreports OPEN Cardiac hypoxic resistance and decreasing lactate during maximum apnea in elite breath hold divers Thomas Kjeld1*, Jakob Møller 1, Kristian Fogh1, Egon Godthaab Hansen2, Henrik Christian Arendrup3, Anders Brenøe Isbrand4, Bo Zerahn4, Jens Højberg5, Ellen Ostenfeld6, Henrik Thomsen1, Lars Christian Gormsen 7 & Marcus Carlsson6 Breath-hold divers (BHD) enduring apnea for more than 4 min are characterized by resistance to release of reactive oxygen species, reduced sensitivity to hypoxia, and low mitochondrial oxygen consumption in their skeletal muscles similar to northern elephant seals. The muscles and myocardium of harbor seals also exhibit metabolic adaptations including increased cardiac lactate-dehydrogenase- activity, exceeding their hypoxic limit. We hypothesized that the myocardium of BHD possesses 15 similar adaptive mechanisms. During maximum apnea O-H2O-PET/CT (n = 6) revealed no myocardial perfusion defcits but increased myocardial blood fow (MBF). Cardiac MRI determined blood oxygen level dependence oxygenation (n = 8) after 4 min of apnea was unaltered compared to rest, whereas cine-MRI demonstrated increased left ventricular wall thickness (LVWT). Arterial blood gases were collected after warm-up and maximum apnea in a pool. At the end of the maximum pool apnea (5 min), arterial saturation decreased to 52%, and lactate decreased 20%. Our fndings contrast with previous MR studies of BHD, that reported elevated cardiac troponins and decreased myocardial 15 perfusion after 4 min of apnea. In conclusion, we demonstrated for the frst time with O-H2O-PET/CT and MRI in elite BHD during maximum apnea, that MBF and LVWT increases while lactate decreases, indicating anaerobic/fat-based cardiac-metabolism similar to diving mammals. -

ACTA BIOMEDICA SUPPLEMENT Atenei Parmensis | Founded 1887
Acta Biomed. - Vol. 91 - Suppl. 1 - February 2020 | ISSN 0392 - 4203 ACTA BIOMEDICA SUPPLEMENT ATENEI PARMENSIS | FOUNDED 1887 Official Journal of the Society of Medicine and Natural Sciences of Parma Acta Biomed. - Vol. 91 - Suppl.1 February 2020 Acta Biomed. - Vol. and Centre on health systems’ organization, quality and sustainability, Parma, Italy The Acta Biomedica is indexed by Index Medicus / Medline Excerpta Medica (EMBASE), the Elsevier BioBASE, Scopus (Elsevier). and Bibliovigilance New insights on upper airway diseases Guest Editors: Giorgio Ciprandi, Desiderio Passali Free on-line www.actabiomedica.it Mattioli 1885 1, comma DCB Parma - Finito di stampare February 2020 46) art. Pubblicazione trimestrale - Poste Italiane s.p.a. - Sped. in A.P. - D.L. 353/2003 (conv. in L. 27/02/2004 n. - D.L. 353/2003 (conv. Pubblicazione trimestrale - Poste Italiane s.p.a. Sped. in A.P. ACTA BIO MEDICA Atenei parmensis founded 1887 OFFICIAL JOURNAL OF THE SOCIETY OF MEDICINE AND NATURAL SCIENCES OF PARMA AND CENTRE ON HEALTH SYSTEM’S ORGANIZATION, QUALITY AND SUSTAINABILITY, PARMA, ITALY free on-line: www.actabiomedica.it EDITOR IN CHIEF ASSOCIATE EDITORS Maurizio Vanelli - Parma, Italy Carlo Signorelli - Parma, Italy Vincenzo Violi - Parma, Italy Marco Vitale - Parma, Italy SECTION EDITORS DEPUTY EDITOR FOR HEALTH DEPUTY EDITOR FOR SERTOT Gianfranco Cervellin- Parma, Italy PROFESSIONS EDITION EDITION Domenico Cucinotta - Bologna, Italy Leopoldo Sarli - Parma, Italy Francesco Pogliacomi - Parma, Italy Vincenzo De Sanctis- Ferrara, Italy Paolo -

Diving Incidents Report Foreward Introduction
APPENDIX A - DIVING INCIDENTS REPORT FOREWARD The 1992 Diving Incidents Report, produced by The British Sub-Aqua Club (BSAC) in the interest of promoting diving safety. As the Goveming Body of the sport of sub-aqua diving within the United Kingdom, the BSAC publishes this information and makes it freely available, subject to the bounds of medical confidentiality, for the benefit of all those concerned with the organisation and conduct of sport diving activities, and in particular, all divers. The BSAC Incidents Reporting Scheme has been established for some 27 years, longer than some diver training organisations. It uses information gathered from a large variety of sources, including the individuals and clubs involved, H.M. Coastguard, recompression chamber operators, the Institute of Naval Medicine and a press cuttings service. All reports received are analysed and summarised in this report with the intention of highlighting any lessons that can be learnt. The BSAC uses this information to identify any trends in diving incidents in order togive its best advice and, if necessary, introduce new training prior to these trends becoming commonplace occurrences. This year (1992) there have been 123 reports received, and it is estimated that over 1.5 million 'man-dives' have been carried out. This is an apparent 30% reduction in dives carried out when compared to 1991 ,possibly due to both the depressed state of the economy and due to the inclement weather on the South Coast for most of the year. The vast majority of these dives were carried out in complete safety and attracted no publicity.