Reynolds American Wants FDA to Ban Vapor E-Cigs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tobacco Securitization
Memorandum Office of Jenine Windeshausen Treasurer-Tax Collector To: The Board of Supervisors From: Jenine Windeshausen, Treasurer-Tax Collector Date: October 27, 2020 Subject: Tobacco Securitization Action Requested a) Adopt a resolution consenting to the issuance and sale by the California County Tobacco Securitization Agency not to exceed $67,000,000 initial principal amount of tobacco settlement bonds (Gold Country Settlement Funding Corporation) Series 2020 Bonds in one or more series and other related matters; authorizing the execution and delivery by the county of a certificate of the county; and authorizing the execution and delivery of and approval of other related documents and actions in connection therewith. b) Direct that eligible proceeds from the Series 2020 Bonds be expended on infrastructure improvements at the Placer County Government Center, construction of the Health and Human Services Building and other Board approved capital facilities projects. Background October 6, 2020 Board of Supervisors Meeting Summary. Your Board received an update regarding the County’s prior tobacco securitizations and information on the potential to refund the Series 2006 Bonds to receive additional proceeds for capital projects. Based on that update, the Board requested the Treasurer to return to the Board on October 27, 2020 with a resolution approving documents and other matters to proceed with refunding the Series 2006 Bonds. In summary from the October 6, 2020 meeting, the County receives annual payments in perpetuity from the 1998 Master Settlement Agreement (MSA). The MSA payments are derived from a percentage of cigarette sales. Placer County issued bonds in 2002 and 2006 to securitize a share of its MSA payments. -

Bases Loaded Beyond E-Cigs and Vapor Devices, New Tobacco Technologies Are Stepping up to by Renée Covino the Plate with Power
JTI Debuts a New Design for Wave MAY/JUNE 2015 VOLUME 18 n NUMBER 3 The Tobacco Innovation Game: BASES LOADED BEYOND e-cigs and vapor devices, NEW tobacco technologies are STEPPING UP to By Renée Covino the plate with POWER. Will reduced-harm products and OTHER innovations change up the COMPETITIVE FIELD? 2015 Vapor Expo Preview The Dark Side of Taxation Uhle Tobacco Company’s Journey PUBLISHER’S LETTER BY ED O’CONNOR Father’s Day 2015… Now 115 Years Old Father’s Day turned 100 years old on June 20, 2010. Sono- So we’ve done the next best thing. Enjoy “Unforbidden ra Smart Dodd, often referred to as the “Mother of Father’s Fruit,” Renee Covino’s article on Cuban cigars appearing in Day,” was 16 years old when her mother died in 1898, the March/April issue of TBI (p. 30) with comments by Dick leaving her father, William Jackson Smart, to raise Sonora Dimeola, a premium cigar expert and long-time friend of and her five younger brothers on a remote farm in eastern the cigar industry, Victor Vitale, Marshall Gray and other ci- Washington. In 1909, Sonora heard a Mother’s Day church gar aficionados. Don’t have a hard copy? Your cigar article sermon inspiring her to propose that fathers receive equal is available to you on tobonline.com. recognition, lending credence to the old saying, “Behind For those of us excited about the dynamic growth of the every good man there’s a good woman.” electronic nicotine (c-cig and VTM) markets, be sure to With the assistance of her pastor, the Spokane, Wash- check out this issue’s Electric Alley column (p. -

Imperial Tobacco Finance
Prospectus Imperial Tobacco Finance PLC A9.4.1.1 (Incorporated with limited liability in England and Wales with registered number 03214426) A9.4.1.2 Imperial Tobacco Finance France SAS (A société par actions simplifiée incorporated in France) €15,000,000,000 Debt Issuance Programme Irrevocably and unconditionally guaranteed by Imperial Tobacco Group PLC A9.4.1.1 (Incorporated with limited liability in England and Wales with registered number 03236483) A9.4.1.2 This Prospectus amends, restates and supersedes the offering circular dated 21st February 2014. Any Notes issued after the date hereof under the Debt Issuance Programme described in this Prospectus (the “Programme”) are issued subject to the provisions set out herein. This Prospectus will not be effective in respect of any Notes issued under the Programme prior to the date hereof. Under the Programme, Imperial Tobacco Finance PLC (“Imperial Finance” or an “Issuer”) and Imperial Tobacco Finance France SAS A6.1 (“Imperial Finance France” or an “Issuer” and together with Imperial Finance “the Issuers”), subject to compliance with all relevant laws, regulations and directives, may from time to time issue debt securities (the “Notes”) guaranteed by Imperial Tobacco Group PLC (“Imperial Tobacco” or the “Guarantor”) and Imperial Tobacco Limited (“ITL”). Please see the Trust Deed dated 6th February 2015 (the “Trust Deed”) which is available for viewing by Noteholders as described on page 124 for further details about the Imperial Tobacco guarantee and page 98 for further details regarding the ITL guarantee. The aggregate nominal amount of Notes outstanding will not at any time exceed €15,000,000,000 (or the equivalent in other currencies). -

E-Cigarettes 003292 Blu Ecigs®
July 12, 2012 TO: Wholesalers reporting Shipment Data to MSAi for blu ecigs SUBJECT: 2012 blu ecigs DATA REPORTING PROGRAMMING DETAILS Enclosed is a copy of the 2012 blu ecigs Data Reporting Programming Details document which includes Data reporting instructions for reporting Electronic Cigarettes to MSAi for blu ecigs using the Multi-CatTM All Tobacco Format. This document is being sent to you in response to your recent enrollment with blu ecigs to report shipment Data for electronic cigarettes. These instructions outline standard requirements for reporting Electronic Cigarette products to blu ecigs, which includes disposables, refill cartridges and kits. The attached document reflects the assignment of a new MSAi Category Code that should be used for reporting Data for Electronic Cigarettes in the Multi-CatTM All Tobacco Format. E-Cigarettes 003292 All Wholesalers reporting Shipment Data to MSAi for blu ecigs must ensure that their systems reflect the required product descriptions to ensure that weekly sales of blu ecigs products to your customers are correctly reported to the Distributor Support Center. Contact the Distributor Support Center at (1-877-544-4429) for questions pertaining to reporting Electronic Cigarettes under the MULTICATTM format. blu ecigs® blu ecigs is a registered trademark of Lorillard Technologies, Inc. blu ecigs Data Reporting Program 2012 MultiCatTM All Tobacco Data Programming Details MSAi Copyright©2012 Updated on 6-28-12 blu ecigs Data Reporting Requirements – V. 1.0 (Effective 7/01/12) blu ecigs DATA REPORTING REQUIREMENTS Data: • All Data which includes shipments to retail, returns, and inventory must be reported in selling units and submitted in the following reporting format outlined in this document: MULTICATTM All Tobacco Format Wholesalers opting to utilize the optional MULTICATTM Format to satisfy blu ecigs reporting program requirements must agree to release all sales Data to blu ecigs. -

Cigarette Price List Effective 02Nd December 2019
Cigarette Price List Effective 02nd December 2019 Price Name Price Name €18.20 B&H Maxi Box 28’s €13.20 Superkings Black €18.20 Silk Cut Blue 28’s €13.20 Superkings Blue €18.20 Silk Cut Purple 28’s €13.20 Superkings Green Menthol €17.00 Marlboro Gold KS Big Box 28s €13.20 Pall Mall 24’s Big Box €16.65 Major 25’s €13.20 JPS Red 24’s €16.40 John Player Blue Big Box 27’s €13.20 JPS Blue 24’s €16.00 Mayfair Superking Original 27’s €13.00 Silk Cut Choice Super Line 20s €16.00 Mayfair Original 27’s €13.00 John Player Blue €16.00 Pall Mall Red 30’s €13.00 John Player Bright Blue €16.00 Pall Mall Blue 30’s €13.00 John Player Blue 100’s €16.00 JPS Blue 29’s €13.00 Lambert & Butler Silver €15.20 Silk Cut Blue 23’s €12.70 B&H Silver 20’s €15.20 Silk Cut Purple 23’s €12.70 B&H Select €15.20 B&H Gold 23’s €12.70 B&H Select 100’s €14.80 John Player Blue Big Box 24’s €12.70 Camel Filters €14.30 Carroll’s Number 1 23 Pack €12.70 Camel Blue €14.20 Mayfair Original 24’s €12.30 Vogue Green €13.70 Players Navy Cut €12.30 Vogue Blue Capsule €13.70 Regal €11.80 Mayfair Double Capsule €13.70 Rothmans €11.80 John Player Blue Compact €13.70 Consulate €11.80 Mayfair Original €13.70 Dunhill International €11.80 Pall Mall Red €13.50 B&H Gold 100’s 20s €11.80 Pall Mall Blue €13.50 Carroll’s No.1 €11.80 Pall Mall Red 100’s €13.50 B&H K.S. -

Volume 34, July 2015
In this issue: VOLUME 34 July 2015 Minneapolis Adopts Restrictions on Flavored Tobacco Products RJ Reynolds and Lorillard Merge Direct Mailing Project Updates TOBACCO MARKETING Reducing Youth Exposure to Tobacco Advertising and Promotion Minneapolis Adopts Restrictions on Flavored UPDATE Tobacco Products By BETSY BROCK The Minneapolis City Council voted unanimously on July 10 to restrict the sale of flavored tobacco products other than menthol to adult-only tobacco shops. The Council also increased the price of cigars to $2.60 apiece. Several other cities in Minnesota, including Maplewood, Bloomington, Saint Paul, and Brooklyn Center, have adopted policies that regulate the price of cheap cigars. However, no other Minnesota cities have restricted the sale of flavored tobacco products. Nationally, New York City and Providence, RI, have similar policies in place that served as a model for the Minneapolis ordinance. The new policy means that only about 15 of the city’s 400-plus tobacco vendors will be allowed to sell candy-flavored tobacco the Minneapolis Youth Congress and the Breathe Free North products. In order to sell these products, the stores must derive at program at NorthPoint Health & Wellness, who said these products least 90 percent of their revenue from tobacco and be adult-only at are appealing to young people. all times. Council Members Cam Gordon (Ward 2) and Blong Yang (Ward 5) “We heard loud and clear from Minneapolis youth that flavored co-authored the ordinance in response to an outcry from youth from tobacco products are what most kids use when they start smoking,” Council Member Cam Gordon said. -

4 Marketing Tactics E-Cigarette Companies Use to Target Youth
NEWS 4 marketing tactics e-cigarette companies use to target youth rom introducing appealing avors to offering college scholarships, manufacturers and sellers of e-cigarettes aggressively f target young people. There are few federal restrictions on e-cigarette marketing, allowing companies to promote their products through traditional outlets — such as TV and radio — despite a ban in 1971 on cigarette advertising on both outlets to reduce cigarette marketing to children. E-cigarette companies also take advantage of other marketing outlets, including the internet, retail environments and recreational venues and events. Youth and young adults are widely exposed to e-cigarette marketing and have high awareness of e-cigarettes, which are the most popular tobacco product among youth. By 2016, nearly 4 out of 5 middle and high school students, or more than 20 million youth, saw at least one e-cigarette advertisement. Here are four ways e-cigarette companies market their products to target young people. 1. Offering scholarships Several e-cigarette companies are offering scholarships, ranging from $250 to $5,000, that involve asking students to write essays on topics like whether vaping could have potential benets, according to the Associated Press. For example, one company asks applicants to write about whether e-cigarettes minimize smoking’s negative effects. E-cigarette manufacturers often say that their products are intended for adults who want to quit smoking; however, the AP reports that, “although some of the scholarships are limited to students 18 and older — the nation’s legal age to buy vaping products — many are open to younger teens or have no age limit.” “Most of these kids are not smokers,” said Robin Koval, CEO and president of Truth Initiative®, in the AP story. -
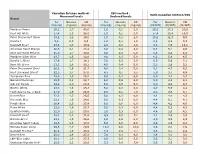
TNCO Levels and Ratio's
Canadian Intense method - ISO method - Ratio Canadian Intense/ISO Measured levels Declared levels Brand Tar Nicotine CO Tar Nicotine CO Tar Nicotine CO (mg/cig) (mg/cig) (mg/cig) (mg/cig) (mg/cig) (mg/cig) (CI/ISO) (CI/ISO) (CI/ISO) Marlboro Prime 26,1 1,7 40,0 1,0 0,1 2,0 26,1 17,2 20,0 Kent HD White 17,4 1,3 28,0 1,0 0,1 2,0 17,4 13,4 14,0 Peter Stuyvesant Silver 15,2 1,2 19,0 1,0 0,1 2,0 15,2 12,3 9,5 Karelia I 9,6 0,9 9,3 1,0 0,1 1,0 9,6 8,6 9,3 Davidoff Blue* 23,6 1,7 30,9 2,9 0,2 2,6 8,3 7,0 12,1 American Spirit Orange 20,5 2,1 18,4 3,0 0,4 4,0 6,8 5,1 4,6 Kent Surround Menthol 25,0 1,7 30,8 4,0 0,4 5,0 6,3 4,3 6,2 Marlboro Silver Blue 24,7 1,5 32,6 4,0 0,3 5,0 6,2 5,0 6,5 Karelia L (Blue) 17,6 1,7 14,1 3,0 0,3 2,0 5,9 5,6 7,1 Kent HD Silver 21,1 1,6 26,2 4,0 0,4 5,0 5,3 3,9 5,2 Peter Stuyvesant Blue* 20,2 1,6 21,7 4,0 0,4 5,0 5,1 4,7 4,3 Kent Surround Silver* 22,5 1,7 27,0 4,5 0,5 5,5 5,0 3,7 4,9 Templeton Blue 25,0 1,8 26,2 5,0 0,4 6,0 5,0 4,4 4,4 Belinda Filterkings 29,9 2,2 24,7 6,0 0,5 6,0 5,0 4,3 4,1 Silk Cut Purple 24,9 2,0 23,4 5,0 0,5 5,0 5,0 4,0 4,7 Boston White 23,3 1,6 25,3 5,0 0,3 6,0 4,7 5,5 4,2 Mark Adams No. -

E-Cigarette Use Among Youth and Young Adults: a Report of the Surgeon General
E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General 2016 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Office of the Surgeon General Rockville, MD National Library of Medicine Cataloging-in-Publication Data Names: United States. Public Health Service. Office of the Surgeon General, issuing body. | National Center for Chronic Disease Prevention and Health Promotion (U.S.). Office on Smoking and Health, issuing body. Title: E-cigarette use among youth and young adults : a report of the Surgeon General. Description: Atlanta, GA : U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. | Includes bibliographical references. Subjects: MESH: Electronic Cigarettes – utilization. | Smoking – adverse effects. | Electronic Cigarettes – adverse effects. | Tobacco Industry. | Young Adult. | Adolescent. | United States. Classification: NLM QV 137 U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health For more information For more information about the Surgeon General’s report, visit www.surgeongeneral.gov. To download copies of this document, go to www.cdc.gov/tobacco. To order copies of this document, go to www.cdc.gov/tobacco and click on Publications Catalog or call 1-800-CDC-INFO (1-800-232-4636); TTY: 1-888-232-6348. Suggested Citation U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S. -

Vapage Community Blog
Vapage Community Blog Call 1 (805) 309-2400 M-F 9-5 PST Account Blog Log In $0.00 Hot!! E-CIGARETTES REFILLS DISPOSABLES VAPORIZERS E-LIQUIDS TANKS New ACCESSORIES ASPIRE SALE! CUSTOMER SERVICE Home Blog Search Vapage Community Blog DISCLAIMER: The views expressed on this blog, represent the personal views of the authors and do not necessarily represent the views or position of Spark Industries, Vapage or Cig2o. Any opinions, recommendations, statements, or other information or content presented or disseminated are those of the respective authors, who are solely responsible for their content. Scientists Stunned After Vaping Test Results! Vaping Indoors is OK? Recent Posts Scientists Shocked After E Cig Test Results!!! Vaping Indoors OK? Scientists Stunned After Over and over again, we’ve heard public health officials argue that Vaping Test Results! we simply do not have enough scientific data to be certain that Vaping Indoors is OK? electronic cigarettes are a safe alternative to tobacco. But the truth is that research from E Cig Test Results abundant and every The FDA And E-Cigarettes: month, we have new studies that point to the truth. The latest Republicans Reject study to hit the scenes is shaming critics and shocking public Amendment To Bill health officials with undeniable evidence from E Cig Test Results Requiring Pre-Market that vamping is safe and effective. Review The new study was published in “Regulatory Toxicology and Pharmacology” and showed what 7 Reason ecigs are a Must exactly Have What to consider when choosing an e-cig The Dos and Don’ts of Handling an E-cig Archives July 2015 May 2015 http://vapage.com/blog/[7/22/15, 2:12:48 PM] Vapage Community Blog April 2015 is hiding in ecig vapor compared to the contents of cigarette smoke. -

Youings Wholesale
CUSTOMER NAME ACCOUNT NO. STOCK RANGE & ORDER BOOK October - December 2018 £3 OFF WHEN YOU BUY 1 x outer of Amber Leaf 30g CPB or 1 x outer of Amber Leaf 30g 3 in 1 CPB Offer available from: 15.10.2018 to 16.12.2018 * £3.00 off Amber Leaf 50g. While stocks last. Limited to 2 outers per customer. Offer open to tobacco buyers only. CIGARETTES, E-CIGARETTES & TOBACCO RANGE • CHRISTMAS 2018 Contents Welcome Contents page I would like to introduce you to my Company. Youings have been supplying tobacco and confectionery for over Cigarettes 6 125 years, a business passed down from father to son through four generations. We therefore have a wealth of experience and knowledge of the trade. The range has Hand Rolling Tobacco 10 broadened over the years to incorporate crisps, snacks, soft drinks, grocery, wines, beers and spirits, coffee and coffee machines. Pipe Tobacco 11 Being a family run business we believe in giving a first class service. With regular calls from our sales team every customer Cigars 12 is known to us personally and not just a number on a computer screen. Whenever there is a need to contact someone in our company he or she should always be able to speak to you. Snuff 13 We consider ourselves to be extremely competitive and offer one of the most extensive ranges you will find in either delivered wholesale or cash and carry. Electronic Cigarettes 14-15 We offer a 3 weekly promotional activity backed by a colour brochure for all categories including grocery and licensed. -

Download E-Cigarettes Industry Marketing And
E-CIGARETTES INDUSTRY MARKETING AND YOUTH TARGETING From introducing appealing flavors to offering college scholarships, manufacturers and sellers of e-cigarettes aggressively target young people. There are few federal restrictions on e-cigarette marketing, allowing companies to promote their products through traditional outlets — such as TV and radio — despite a ban in 1971 on cigarette E-liquid Food product E-liquid Food product advertising in both outlets to reduce cigarette marketing to children. E-cigarette companies also take advantage of other marketing outlets, including the internet, retail environments, and recreational venues and events. E-cigarettes are the most popular tobacco E-liquid Food product E-liquid Food product product among youth, with about one in five high and Drug Administration U.S. Food Photo: 1 school students using e-cigarettes in 2020. E-cigarettes capitalize on offering Youth and young adults are widely exposed to e-cigarette marketing and have high awareness many kid-friendly food flavors, of the products. Among middle and high school students who reported contact with a potential such as mint, cotton candy source of tobacco advertising in 2019, such as and gummy bear. going to a convenience store or gas station, watching television, or reading magazines, nearly 70% (69.3%) were exposed to e-cigarette marketing.22 MARKETING TACTICS In January 2020, the FDA prioritized enforcement > People most often heard about e-cigarettes against the sale of most candy or fruit flavors through in-person communications, by in “closed pod” refillable e-cigarettes, like the seeing them for sale and through online and popular brand JUUL, but exempted disposable television advertisements, in which some 3-7 flavored e-cigarettes including newer products celebrities endorsed the products.