Tobacco Securitization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I UNITED STATES DISTRICT COURT for the DISTRICT OF
Case 1:99-cv-02496-PLF Document 6276 Filed 08/03/18 Page 1 of 59 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA ____________________________________ UNITED STATES OF AMERICA, ) Plaintiff, ) Civil Action No. 99-CV-2496 (PLF) ) and ) ) CAMPAIGN FOR TOBACCO-FREE ) KIDS, et al., ) Plaintiff-Intervenors, ) ) v. ) ) PHILIP MORRIS USA INC., et al., ) Defendants. ) ) and ) ) ITG BRANDS, LLC, et al., ) Post-Judgment Parties ) Regarding Remedies ) PLAINTIFFS’ 2018 SUPPLEMENTAL BRIEF ON RETAIL POINT OF SALE REMEDY TABLE OF CONTENTS TABLE OF AUTHORITIES ......................................................................................................... iv INTRODUCTION .......................................................................................................................... 1 FACTUAL BACKGROUND ......................................................................................................... 5 I. The Deal between Cigarette Manufacturers and Retailers ............................................... 5 II. Benefits to Participating Retailers .................................................................................... 6 III. Manufacturers’ Contractual Control over Space for Cigarette Marketing and Promotional Displays ....................................................................................................... 8 A. The types of retail advertising and marketing space ........................................8 B. The manufacturers’ contractual authority over participating retailers’ space 10 1. The -

South Carolina Tobacco Directory
South Carolina Tobacco Directory Updated: June 14, 2019 Office of the Attorney General South Carolina Tobacco Directory Alan Wilson Company Name Brand Name Original Certification Date Agreement type Status Cheyenne International LLC Decade 8/10/2005 NPM Compliant Aura 6/16/2014 NPM Compliant Cheyenne 8/10/2005 NPM Compliant Commonwealth Brands, Inc. USA Gold 8/10/2005 PM Compliant Crowns 3/16/2011 PM Compliant Rave 7/15/2009 PM Compliant Rave (RYO) 7/15/2009 PM Compliant Montclair 8/10/2005 PM Compliant Fortuna 9/15/2008 PM Compliant Sonoma 8/10/2005 PM Compliant Compania Tabacalera Internacional, S.A. Director 12/27/2017 NPM Compliant Dosal Tobacco Corporation 305 8/9/2010 NPM Compliant DTC 8/9/2010 NPM Compliant Firebird Manufacturing Cherokee 8/4/2010 NPM Compliant Palmetto 8/4/2010 NPM Compliant ITG Brands LLC Kool 8/12/2005 PM Compliant Winston 8/12/2005 PM Compliant Salem 8/12/2005 PM Compliant Maverick 8/11/2005 PM Compliant Japan Tobacco International U.S.A., Inc. Wave 8/10/2005 PM Compliant LD by L. Ducat 5/6/2016 PM Compliant Export A 8/10/2005 PM Compliant Kretek International Taj Mahal Bidis 10/18/2005 PM Compliant KT&G Corporation page: 0 of 1 Carnival 2/15/2012 NPM Compliant THIS 2/15/2018 NPM Compliant Timeless Time 2/15/2012 NPM Compliant Liggett Group Inc. Pyramid 8/9/2005 PM Compliant Liggett Select 8/9/2005 PM Compliant Eve 8/9/2005 PM Compliant Bronson 10/4/2011 PM Compliant Grand Prix 8/9/2005 PM Compliant Tourney 9/8/2005 PM Compliant Tourney Slims 8/9/2005 PM Compliant NASCO Products, LLC SF 1/5/2015 PM Compliant Native Trading Associates Mohawk 8/6/2013 NPM Compliant Native 6/14/2006 NPM Compliant Native (RYO) 12/10/2007 NPM Compliant Ohserase Manufacturing Signal 8/1/2011 NPM Compliant Peter Stokkebye Tobaksfabrik A/S Turkish Export (RYO) 8/15/2013 PM Compliant Danish Export (RYO) 8/15/2013 PM Compliant London Export (RYO) 8/15/2013 PM Compliant Amsterdam Shag (RYO) 8/15/2013 PM Compliant Stockholm Blend (RYO) 8/15/2013 PM Compliant Norwegian Shag (RYO) 8/15/2013 PM Compliant Philip Morris USA Inc. -

Empty Discarded Pack Data and the Prevalence of Illicit Trade in Cigarettes in California
Pepperdine University Pepperdine Digital Commons School of Public Policy Working Papers School of Public Policy 1-22-2019 Empty Discarded Pack Data and the Prevalence of Illicit Trade in Cigarettes in California James Prieger Pepperdine University, [email protected] Follow this and additional works at: https://digitalcommons.pepperdine.edu/sppworkingpapers Part of the Econometrics Commons, Health Economics Commons, Political Economy Commons, and the Public Affairs, Public Policy and Public Administration Commons Recommended Citation Prieger, James, "Empty Discarded Pack Data and the Prevalence of Illicit Trade in Cigarettes in California" (2019). Pepperdine University, School of Public Policy Working Papers. Paper 75. https://digitalcommons.pepperdine.edu/sppworkingpapers/75 This Article is brought to you for free and open access by the School of Public Policy at Pepperdine Digital Commons. It has been accepted for inclusion in School of Public Policy Working Papers by an authorized administrator of Pepperdine Digital Commons. For more information, please contact [email protected], [email protected], [email protected]. PEPPERDINE UNIVERSITY Empty Discarded Pack Data and the Prevalence of Illicit Trade in Cigarettes in California January 22, 2019 Jonathan Kulick James E. Prieger JDK Analysis Professor 30 Regent #318 Pepperdine University Jersey City, NJ 07302 School of Public Policy 24255 Pacific Coast Highway [email protected] Malibu, CA 90263-7490 James.Prieger@ pepperdine.edu School of Public Policy 24255 Pacific Coast Highway Malibu, CA 90263- 7490 Abstract Illicit trade in tobacco products (ITTP) creates many harms including reduced tax revenues; damages to the economic interests of legitimate actors; funding for organized-crime and terrorist groups; negative effects of participation in illicit markets, such as violence and incarceration; and reduced effectiveness of smoking-reduction policies, leading to increased damage to health. -

Cigarette Company Paid for Lung Cancer Study - New York Times
Cigarette Company Paid for Lung Cancer Study - New York Times http://www.nytimes.com/2008/03/26/health/research/26lung.html?_r=1&sq=ct%20scans%20... March 26, 2008 Cigarette Company Paid for Lung Cancer Study By GARDINER HARRIS In October 2006, Dr. Claudia Henschke of Weill Cornell Medical College jolted the cancer world with a study saying that 80 percent of lung cancer deaths could be prevented through widespread use of CT scans. Small print at the end of the study, published in The New England Journal of Medicine, noted that it had been financed in part by a little-known charity called the Foundation for Lung Cancer: Early Detection, Prevention & Treatment. A review of tax records by The New York Times shows that the foundation was underwritten almost entirely by $3.6 million in grants from the parent company of the Liggett Group, maker of Liggett Select, Eve, Grand Prix, Quest and Pyramid cigarette brands. The foundation got four grants from the Vector Group, Liggett’s parent, from 2000 to 2003. Dr. Jeffrey M. Drazen, editor in chief of the medical journal, said he was surprised. “In the seven years that I’ve been here, we have never knowingly published anything supported by” a cigarette maker, Dr. Drazen said. An increasing number of universities do not accept grants from cigarette makers, and a growing awareness of the influence that companies can have over research outcomes, even when donations are at arm’s length, has led nearly all medical journals and associations to demand that researchers accurately disclose financing sources. -

Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii
Hawaii State Fire Council Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii Submitted to The Twenty-Eighth State Legislature Regular Session June 2015 2014 Reduced Ignition Propensity Cigarette Report to the Hawaii State Legislature Table of Contents Executive Summary .…………………………………………………………………….... 4 Purpose ..………………………………………………………………………....................4 Mission of the State Fire Council………………………………………………………......4 Smoking-Material Fire Facts……………………………………………………….............5 Reduced Ignition Propensity Cigarettes (RIPC) Defined……………………………......6 RIPC Regulatory History…………………………………………………………………….7 RIPC Review for Hawaii…………………………………………………………………….9 RIPC Accomplishments in Hawaii (January 1 to June 30, 2014)……………………..10 RIPC Future Considerations……………………………………………………………....14 Conclusion………………………………………………………………………….............15 Bibliography…………………………………………………………………………………17 Appendices Appendix A: All Cigarette Fires (State of Hawaii) with Property and Contents Loss Related to Cigarettes 2003 to 2013………………………………………………………18 Appendix B: Building Fires Caused by Cigarettes (State of Hawaii) with Property and Contents Loss 2003 to 2013………………………………………………………………19 Appendix C: Cigarette Related Building Fires 2003 to 2013…………………………..20 Appendix D: Injuries/Fatalities Due To Cigarette Fire 2003 to 2013 ………………....21 Appendix E: HRS 132C……………………………………………………………...........22 Appendix F: Estimated RIPC Budget 2014-2016………………………………...........32 Appendix G: List of RIPC Brands Being Sold in Hawaii………………………………..33 2 2014 -

Altria Group, Inc. Annual Report
Altria Group, Inc. 2019 Annual Report an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company an Altria Company Howard A. Willard III Dear Fellow Shareholders Chairman of the Board and CEO Altria delivered solid performance in a dynamic year for the tobacco industry. Our core tobacco businesses delivered outstanding financial performance, and we made significant progress advancing our non-combustible product platform. We believe Altria’s enhanced business platform positions us well for future success. 2019 Highlights n Grew adjusted diluted earnings per share (EPS) by 5.8%, primarily driven by our core tobacco businesses; and types of legal cases pending against it, especially during the fourth n Achieved $600 million in annualized cost savings, exceeding our $575 quarter of the year. Altria recorded two impairment charges of our JUUL million target announced in December 2018; asset in 2019, reducing our investment to $4.2 billion at year-end, down from n Increased our regular quarterly dividend for the 54th time in 50 years $12.8 billion, our 2019 initial investment. JUUL remains the U.S. leader in the and paid shareholders approximately $6.1 billion in dividends; and e-vapor category, and in January 2020 we revised certain terms governing n Repurchased 16.5 million Altria shares for a total cost of $845 million. -

Directory for Cigarettes and Roll-Your-Own Tobacco
DEPARTMENT OF THE ATTORNEY GENERAL STATE OF HAWAI‘I DIRECTORY FOR CIGARETTES AND ROLL-YOUR-OWN TOBACCO of (Available at: http://ag.hawaii.gov/cjd/tobacco-enforcement-unit/) Certified Tobacco Product Manufacturers Their Brands and Brand Families DEPARTMENT OF THE ATTORNEY GENERAL TOBACCO ENFORCEMENT UNIT 425 QUEEN STREET HONOLULU, HAWAI‘I 96813 (808) 586-1203 INDEX I. Directory: Cigarettes and Roll-Your-Own Tobacco Page 1. INTRODUCTION 3 2. DEFINITIONS 3 3. NOTICES 5 II. Update Summary For September 22, 2017 Posting III. Alphabetical Brand List IV. Compliant Participating Manufacturers List V. Compliant Non-Participating Manufacturers List Posted: September 22, 2017 2 1. INTRODUCTION Pursuant to Haw. Rev. Stat. §245-22.5(a), beginning December 1, 2003, it shall be unlawful for an entity (1) to affix a stamp to a package or other container of cigarettes belonging to a tobacco product manufacturer or brand family not included in this directory, or (2) to import, sell, offer, keep, store, acquire, transport, distribute, receive, or possess for sale or distribution cigarettes1 belonging to a tobacco product manufacturer or brand family not included in this directory. Pursuant to §245-22.5(b), any entity that knowingly violates subsection (a) shall be guilty of a class C felony. Pursuant to Haw. Rev. Stat. §§245-40 and 245-41, any cigarettes unlawfully possessed, kept, stored, acquired, transported, or sold in violation of Haw. Rev. Stat. §245-22.5 may be ordered forfeited pursuant to Haw. Rev. Stat., Chapter 712A. In addition, the attorney general may apply for a temporary or permanent injunction restraining any person from violating or continuing to violate Haw. -

Altria Group, Inc. Annual Report
ananan Altria Altria Altria Company Company Company an Altria Company ananan Altria Altria Altria Company Company Company | Inc. Altria Group, Report 2020 Annual an Altria Company From tobacco company To tobacco harm reduction company ananan Altria Altria Altria Company Company Company an Altria Company ananan Altria Altria Altria Company Company Company an Altria Company Altria Group, Inc. Altria Group, Inc. | 6601 W. Broad Street | Richmond, VA 23230-1723 | altria.com 2020 Annual Report Altria 2020 Annual Report | Andra Design Studio | Tuesday, February 2, 2021 9:00am Altria 2020 Annual Report | Andra Design Studio | Tuesday, February 2, 2021 9:00am Dear Fellow Shareholders March 11, 2021 Altria delivered outstanding results in 2020 and made steady progress toward our 10-Year Vision (Vision) despite the many challenges we faced. Our tobacco businesses were resilient and our employees rose to the challenge together to navigate the COVID-19 pandemic, political and social unrest, and an uncertain economic outlook. Altria’s full-year adjusted diluted earnings per share (EPS) grew 3.6% driven primarily by strong performance of our tobacco businesses, and we increased our dividend for the 55th time in 51 years. Moving Beyond Smoking: Progress Toward Our 10-Year Vision Building on our long history of industry leadership, our Vision is to responsibly lead the transition of adult smokers to a non-combustible future. Altria is Moving Beyond Smoking and leading the way by taking actions to transition millions to potentially less harmful choices — a substantial opportunity for adult tobacco consumers 21+, Altria’s businesses, and society. To achieve our Vision, we are building a deep understanding of evolving adult tobacco consumer preferences, expanding awareness and availability of our non-combustible portfolio, and, when authorized by FDA, educating adult smokers about the benefits of switching to alternative products. -

An Overview of Industry Investments, Impact and Influence in the Former Soviet Union a B Gilmore, M Mckee
136 Tob Control: first published as 10.1136/tc.2002.002667 on 2 June 2004. Downloaded from RESEARCH PAPER Tobacco and transition: an overview of industry investments, impact and influence in the former Soviet Union A B Gilmore, M McKee ............................................................................................................................... Tobacco Control 2004;13:136–142. doi: 10.1136/tc.2002.002667 Objectives: To quantify the contribution the tobacco industry has made to foreign direct investment (FDI) in the former Soviet Union (FSU) as an indicator of its political and economic leverage; to explore the impact this has had on production capacity and tobacco control in the region. Design: Data on industry investment and its impact on cigarette production capacity were collated from industry journals, reports, and websites. Data on total FDI were obtained from the European Bank of See end of article for Reconstruction and Development. authors’ affiliations Results: By the end of 2000, transnational tobacco companies (TTCs) had invested over US$2.7 billion in ....................... 10 countries of the FSU. Tobacco money as a proportion of FDI varies from 1% to over 30% in Uzbekistan. Correspondence to: Cigarette production capacity in the factories receiving investments tripled from 146 to 416 billion Dr A Gilmore, European cigarettes per annum and the TTCs’ market share has increased from nothing to between 50–100% in the Centre on Health of Societies in Transition, markets in which they invested. Findings suggest that the effectiveness of national tobacco control London School of Hygiene measures corresponds broadly to the nature of the political and economic transition in each country and and Tropical Medicine, the size of industry investment, which is determined in part by the political context. -

Directory of Fire Safe Certified Cigarette Brand Styles Updated 11/20/09
Directory of Fire Safe Certified Cigarette Brand Styles Updated 11/20/09 Beginning August 1, 2008, only the cigarette brands and styles listed below are allowed to be imported, stamped and/or sold in the State of Alaska. Per AS 18.74, these brands must be marked as fire safe on the packaging. The brand styles listed below have been certified as fire safe by the State Fire Marshall, bear the "FSC" marking. There is an exception to these requirements. The new fire safe law allows for the sale of cigarettes that are not fire safe and do not have the "FSC" marking as long as they were stamped and in the State of Alaska before August 1, 2008 and found on the "Directory of MSA Compliant Cigarette & RYO Brands." Filter/ Non- Brand Style Length Circ. Filter Pkg. Descr. Manufacturer 1839 Full Flavor 82.7 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Full Flavor 97 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Full Flavor 83 24.60 Non-Filter Soft Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Light 83 24.40 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Light 97 24.50 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol 97 24.50 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol 83 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol Light 83 24.50 Filter Hard Pack U.S. -
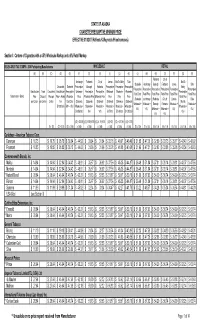
Min Price 1-2-07
STATE OF ALASKA CIGARETTE PRESUMPTIVE MINIMUM PRICE EFFECTIVE 01/02/07 (Reflects RJReynolds Price Increases) Section I: Cartons of Cigarettes with a 4.5% Wholesale Markup and a 6% Retail Markup BLUE & GRAY TAX STAMPS - 2006 Participating Manufacturers WHOLESALE RETAIL (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) (O) (P) (Q) (R) Fairbanks City of Anchorage Fairbanks City of Juneau Mat-Su Valley Sitka Mat-Su Statewide Anchorage Borough Fairbanks Juneau Sitka Discounted Statewide Presumptive Borough Fairbanks Presumptive Presumptive Presumptive Valley Presumptive Presumptive Presumptive Presumptive Presumptive Presumptive Manufacturer Trade Discounted Manufacturer Presumptive Wholesale Presumptive Presumptive Wholesale Wholesale Wholesale Presumptive Retail Cost Retail Price Retail Price Retail Price Retail Price Retail Price Manufacturer / Brand Price Discount Price per Price + Alaska Wholesale Price Wholesale Price Wholesale Price Price Price Price Retail Price (Statewide (Anchorage (Fairbanks (City of (Juneau (Sitka per Carton per Carton Carton Tax Cost (Disc (Statewide (Statewide (Statewide (Statewide (Statewide (Statewide (Mat-Su Wholesale + Wholesale + Borough Fairbanks Wholesale + Wholesale + ($18.00/ctn) Mfr + 4.5%) Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + 6%) 6%) Wholesale + Wholesale + 6%) 6%) $13.86/ctn) 8%) 16%) $3.00/ctn) $10.46/ctn) $10.00/ctn) 6%) 6%) 6%) ((D) + $13.86) (((C)x1.08)+$18) (((C)x1.16)+$18) ((D)+$3) ((D) + $10.46) ((D) + $10) (A) - (B) (C) + $18 (D) x 1.045 x 1.045 x 1.045 x 1.045 x 1.045 x 1.045 x1.045 (E) x 1.06 (F) x 1.06 (G) x 1.06 (H) x 1.06 (I) x 1.06 (J) x 1.06 (K) x 1.06 Caribbean - American Tobacco Corp. -

Cigarettes and Tobacco Products Removed from the California Tobacco Directory by Brand
Cigarettes and Tobacco Products Removed From The California Tobacco Directory by Brand Brand Manufacturer Date Comments Removed #117 - RYO National Tobacco Company 10/21/2011 5/6/05 Man. Change from RBJ to National Tobacco Company 10/20's (ten-twenty's) M/s Dhanraj International 2/6/2012 2/2/05 Man. Name change from Dhanraj Imports, Inc. 10/20's (ten-twenty's) - RYO M/s Dhanraj International 2/6/2012 1st Choice R.J. Reynolds Tobacco Company 5/3/2010 Removed 5/2/08; Reinstated 7/11/08 32 Degrees General Tobacco 2/28/2010 4 Aces - RYO Top Tobacco, LP 11/12/2010 A Touch of Clove Sherman 1400 Broadway N.Y.C. Inc. 9/25/2009 AB Rimboche' - RYO Daughters & Ryan, Inc. 6/18/2010 Ace King Maker Marketing 5/21/2020 All American Value Philip Morris, USA 5/5/2006 All Star Liberty Brands, LLC 5/5/2006 Alpine Philip Morris, USA 8/14/2013 Removed 5/4/07; Reinstated 5/8/09 Always Save Liberty Brands, LLC 5/4/2007 American R.J. Reynolds Tobacco Company 5/6/2005 American Bison Wind River Tobacco Company, LLC 9/22/2015 American Blend Mac Baren Tobacco Company 5/4/2007 American Harvest Sandia Tobacco Manufacturers, Inc. 8/31/2016 American Harvest - RYO Truth & Liberty Manufacturing 8/2/2016 American Liberty Les Tabacs Spokan 5/12/2006 Amphora - RYO Top Tobacco, LP 11/18/2011 Andron's Passion VCT 5/4/2007 Andron's Passion VCT 5/4/2007 Arango Sportsman - RYO Daughters & Ryan, Inc. 6/18/2010 Arbo - RYO VCT 5/4/2007 Ashford Von Eicken Group 5/8/2009 Ashford - RYO Von Eicken Group 12/23/2011 Athey (Old Timer's) Daughters & Ryan, Inc.