MRI Findings of Intrinsic and Extrinsic Duodenal Abnormalities And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of Major Duodenal Papilla Influences ERCP Outcomes
Journal of Clinical Medicine Article Anatomy of Major Duodenal Papilla Influences ERCP Outcomes and Complication Rates: A Single Center Prospective Study Gheorghe G. Balan 1 , Mukul Arya 2, Adrian Catinean 3, Vasile Sandru 4,*, Mihaela Moscalu 1 , Gabriel Constantinescu 5, Anca Trifan 1 , Gabriela Stefanescu 1,* and Catalin Victor Sfarti 1 1 Faculty of Medicine, Grigore T. Popa University of Medicine and Pharmacy, 700115 Iasi, Romania; [email protected] (G.G.B.); [email protected] (M.M.); [email protected] (A.T.); [email protected] (C.V.S.) 2 New York Presbitarian Brooklyn Methodist Hospital, New York, NY 11215, USA; [email protected] 3 Faculty of Medicine, Iuliu Hatieganu University of Medicine and Pharmacy, 400012 Cluj-Napoca, Romania; [email protected] 4 Department of Gastroenterology, Clinical Emergency Hospital of Bucharest, 014461 Bucharest, Romania 5 Faculty of Medicine, Carol Davila University of Medicine and Pharmacy, 020021 Bucharest, Romania; [email protected] * Correspondence: [email protected] (V.S.); [email protected] (G.S.) Received: 27 March 2020; Accepted: 25 May 2020; Published: 28 May 2020 Abstract: Background: Endoscopic retrograde cholangiopancreatography (ERCP) has been one of the most intensely studied endoscopic procedures due to its overall high complication rates when compared to other digestive endoscopy procedures. The safety and outcome of such procedures have been linked to multiple procedure- or patient-related risk factors. The aim of our study is to evaluate whether the morphology of the major duodenal papilla influences the ERCP outcomes and complication rates. Methods: A total of 322 patients with a native papilla have been included in the study over an eight month period. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Small & Large Intestine
Small & Large Intestine Gastrointestinal block-Anatomy-Lecture 6,7 Editing file Objectives Color guide : Only in boys slides in Green Only in girls slides in Purple important in Red At the end of the lecture, students should be able to: Notes in Grey ● List the different parts of small intestine. ● Describe the anatomy of duodenum, jejunum & ileum regarding: (the shape, length, site of beginning & termination, peritoneal covering, arterial supply & lymphatic drainage) ● Differentiate between each part of duodenum regarding the length, level & relations. ● Differentiate between the jejunum & ileum regarding the characteristic anatomical features of each of them. ● List the different parts of large intestine. ● List the characteristic features of colon. ● Describe the anatomy of different parts of large intestine regarding: (the surface anatomy, peritoneal covering, relations, arterial & nerve supply) Small intestine The small intestine divided into : Fixed Part (No Mesentery): Free (Movable) Part (With Parts Duodenum* Mesentery): Jejunum & Ileum Shape C-shaped loop coiled tube Length 10 inches 6 meters (20 feet) Transverse Colon separates the Beginning At pyloro-duodenal junction at duodeno-jejunal flexure stomach/liver from the jejunum/ileum Termination At duodeno-jejunal flexure at ileo-ceacal flexure Peritoneal Covering Retroperitoneal mesentery of small intestine Divisions 4 parts --------- Foregut (above bile duct opening in 2nd part )& Midgut Embryological origin Midgut (below bile duct opening in 2nd part) So 2nd part has double -

Digestive System
Naziha Sultan Ahmed, BVMS, MSc Scientific degree (Assis. Prof.), Department of Anatomy College of Veterinary Medicine, University of Mosul, Mosul, Iraq https://orcid.org/0000-0002-2856-8277 https://www.researchgate.net/profile/Naziha_Ahmed Anatomy | Part 18| 2nd year 2019 Digestive System Fixation of the liver (Ligaments of the liver): 1-Lesser omentum: consist of two parts: a/Hepatogastric ligament: connect between hepatic porta and lesser curvature of the stomach . b/Hepatoduodenal ligament: connect between hepatic porta and the cranial part of the duodenum. 2-Coronary ligament: connect between the parietal (diaphragmatic ) surface of liver, with the diaphragm and the caudal vena cava. 3-Falciform ligament: connect between the notch of round ligament in the liver and the sternal part of the diaphragm. 4-Round ligament: the residue (remnants) of the umbilical vein of the fetus. 5-Hepatorenal ligament: connect between the right lobe of liver and the right kidney. 6-Right & left triangular ligaments: connect between the dorsal border of right and left lobes of the liver and the diaphragm . Both ligaments continue medially with the coronary ligament. CouAnatomy | Digestive system | Assis. Prof. Naziha Sultan Ahmed Page | 1 The pancreas: Pancreas has V-shape. It consists of base and two limbs (right & left limbs). *In horse: large pancreas body perforated by portal vein and long left limb, with short right limb (because of large size of cecum in horse ). The horse pancreas has two ducts: 1-Chief pancreatic duct: opens with bile duct at the major duodenal papilla. 2-Accessory pancreatic duct: opens at the minor duodenal papilla. *In dog: pancreas notched by the portal vein. -

Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and Main Principals Of
Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and main principals of anatomical, pharmaceutical and clinical terminology (Student's book) Simferopol, 2017 Contents No. Topics Page 1. UNIT I. Latin language history. Phonetics. Alphabet. Vowels and consonants classification. Diphthongs. Digraphs. Letter combinations. 4-13 Syllable shortness and longitude. Stress rules. 2. UNIT II. Grammatical noun categories, declension characteristics, noun 14-25 dictionary forms, determination of the noun stems, nominative and genitive cases and their significance in terms formation. I-st noun declension. 3. UNIT III. Adjectives and its grammatical categories. Classes of adjectives. Adjective entries in dictionaries. Adjectives of the I-st group. Gender 26-36 endings, stem-determining. 4. UNIT IV. Adjectives of the 2-nd group. Morphological characteristics of two- and multi-word anatomical terms. Syntax of two- and multi-word 37-49 anatomical terms. Nouns of the 2nd declension 5. UNIT V. General characteristic of the nouns of the 3rd declension. Parisyllabic and imparisyllabic nouns. Types of stems of the nouns of the 50-58 3rd declension and their peculiarities. 3rd declension nouns in combination with agreed and non-agreed attributes 6. UNIT VI. Peculiarities of 3rd declension nouns of masculine, feminine and neuter genders. Muscle names referring to their functions. Exceptions to the 59-71 gender rule of 3rd declension nouns for all three genders 7. UNIT VII. 1st, 2nd and 3rd declension nouns in combination with II class adjectives. Present Participle and its declension. Anatomical terms 72-81 consisting of nouns and participles 8. UNIT VIII. Nouns of the 4th and 5th declensions and their combination with 82-89 adjectives 9. -
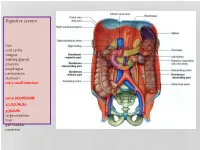
Digestive System
Digestive system Lips oral cavity tongue salivary glands pharynx esophagus peritoneum stomach m6-7 small intestine: cm25 DEODENUM: 2/5JEJUNUM: 3/5ILIUM: large intestine liver gall bladder pancreas :Duodenum Sup. Part Descending part Horizontal part Ascending part Major duodenal papilla Minor duodenal papilla : JEJUNUM / ILIUM From duodenojejunal curvature To Iliocecal Different jejunum & ilium 3Tenia coli: sigmoid 2 / rectum 0 Houstra / saccule Appendices epiploicae : No in Appendix / cecum / rectum Many in sigmoid DIFFERENT BETWEEN SMALL AND LARGE INTESTINE large intestine 1.5m Cecum appendix ascending colon transverse colon descending colon sigmoid colon rectum anal canal McBurneys point ascending colon transverse colon descending colon sigmoid colon :Rectum cm 12 Sacral flexure Puborectalis muscle Perineal flexure Rectum Ampulla : Anal canal cm 4 sup. = anal column / anal valve / anal sinus / pectinate line 2/3 Hiltons white line .inf 1/3 :Anal sphincter Internal sphincter External sphincter: deep / superficial / subcutaneous Liver: 1.5 kgr Located in Rt. & lf. Hypochondriac & epigastric region Surfaces: Sup. Anterior Surface: falciform ligament Inf. Surface: H shape fissure / porta hepatis / quadrate lobe / qudate lobe Post. Surface: bare area Rt. Surface: ribs 7-11 / Rt. lung Liver viewed from posterior Liver viewed from inferior Liver viewed from inferior Liver viewed from posterior :Liver vasculature 20%Hepatic artery 80%Portal vein Supra hepatic vein IVC :Nerve Sympathetic Parasympathetic From Vagus+ Phernic + celiac :Gall bladder Located in inf. Surface of liver cm7-10 Length: cm3-4 Wide: :Structure Fundus Body Infundibulum Neck Systic duct :Pancreas 2–L1 L cm15-20 Length: cm3Wide: cm2 Thickness: gram 90 :Structure Head :Uncinate process / sup. Mesenteric artery Neck: portal vein / sup. -

The Gallbladder
The Gallbladder Anatomy of the gallbladder Location: Right cranial abdominal quadrant. In the gallbladder fossa of the liver. o Between the quadrate and right medial liver lobes. Macroscopic: Pear-shaped organ Fundus, body and neck. o Neck attaches, via a short cystic duct, to the common bile duct. Opens into the duodenum via sphincter of Oddi at the major duodenal papilla. Found on the mesenteric margin of orad duodenum. o 3-6 cm aboral to pylorus. 1-2cm of distal common bile duct runs intramural. Species differences: Dogs: o Common bile duct enters at major duodenal papilla. Adjacent to pancreatic duct (no confluence prior to entrance). o Accessory pancreatic duct enters at minor duodenal papilla. ± 2 cm aboral to major duodenal papilla. MAJOR conduit for pancreatic secretions. Cats: o Common bile duct and pancreatic duct converge before opening at major duodenal papilla. Thus, any surgical procedure that affects the major duodenal papilla can affect the exocrine pancreatic secretions in cats. o Accessory pancreatic duct only seen in 20% of cats. 1 Gallbladder wall: 5 histologically distinct layers. From innermost these include: o Epithelium, o Submucosa (consisting of the lamina propria and tunica submucosa), o Tunica muscularis externa, o Tunica serosa (outermost layer covers gallbladder facing away from the liver), o Tunica adventitia (outermost layer covers gallbladder facing towards the liver). Blood supply: Solely by the cystic artery (derived from the left branch of the hepatic artery). o Susceptible to ischaemic necrosis should its vascular supply become compromised. Function: Storage reservoir for bile o Concentrated (up to 10-fold), acidified (through epithelial acid secretions) and modified (by the addition of mucin and immunoglobulins) before being released into the gastrointestinal tract at the major duodenal. -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

Location of Major Duodenal Papilla in Human Duodenum Sharmina Sayeed1, Shamim Ara2, Mesbahul Hoque3, Zannatul Ferdous4, Kanetarin Kashem5
Bangladesh Journal of Anatomy January 2014, Vol. 12, No. 1 pp. 22-24 Location of Major Duodenal Papilla in Human Duodenum Sharmina Sayeed1, Shamim Ara2, Mesbahul Hoque3, Zannatul Ferdous4, Kanetarin Kashem5 Abstract Context: The major duodenal papilla is one of the most fascinating papilla present at the duodenum attracting many gastroenterologists as they do endoscopic retrograde cholangiopancreatiography (ERCP) for diagnosis and treatment purpose of many diseases. Most of the textbooks of Anatomy describe that the summit of major duodenal papilla is situated posteromedially in the descending part of duodenum. Henceforth the present study was undertaken in 70 human duodenums to observe the location of major duodenal papilla. Materials & Methods: A cross-sectional observational study was conducted in the department of Anatomy, Dhaka Medical College, Dhaka from July 2010 to June 2011. Seventy postmortem human duodenums were collected from unclaimed dead bodies that were under examination in the morgue of department of Forensic Medicine and the department of Anatomy of Dhaka Medical College, Dhaka. Location of major duodenal papilla was observed and recorded. Results: The location of major duodenal papilla was observed in the medial wall of second part of duodenum in 78.6% specimens, in the posteromedial wall of second part in 15.7% cases and in the posteromedial wall of the junction between second and third part in 4.3% and absent in 1.4% duodenum. Conclusion: The location of major duodenal papilla varies in position. Key words: Major duodenal papilla. Introduction Location of major duodenal papilla provides The major duodenal papilla (papilla of Vater) is the information to gastroenterologist regarding opening point where the dilated junction of the pancreatic of common bile duct and pancreatic duct in the duct and the bile duct (ampulla of Vater) enters the duodenum during ERCP. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

Chronic Pancreatitis: Introduction
Chronic Pancreatitis: Introduction Authors: Anthony N. Kalloo, MD; Lynn Norwitz, BS; Charles J. Yeo, MD Chronic pancreatitis is a relatively rare disorder occurring in about 20 per 100,000 population. The disease is progressive with persistent inflammation leading to damage and/or destruction of the pancreas . Endocrine and exocrine functional impairment results from the irreversible pancreatic injury. The pancreas is located deep in the retroperitoneal space of the upper part of the abdomen (Figure 1). It is almost completely covered by the stomach and duodenum . This elongated gland (12–20 cm in the adult) has a lobe-like structure. Variation in shape and exact body location is common. In most people, the larger part of the gland's head is located to the right of the spine or directly over the spinal column and extends to the spleen . The pancreas has both exocrine and endocrine functions. In its exocrine capacity, the acinar cells produce digestive juices, which are secreted into the intestine and are essential in the breakdown and metabolism of proteins, fats and carbohydrates. In its endocrine function capacity, the pancreas also produces insulin and glucagon , which are secreted into the blood to regulate glucose levels. Figure 1. Location of the pancreas in the body. What is Chronic Pancreatitis? Chronic pancreatitis is characterized by inflammatory changes of the pancreas involving some or all of the following: fibrosis, calcification, pancreatic ductal inflammation, and pancreatic stone formation (Figure 2). Although autopsies indicate that there is a 0.5–5% incidence of pancreatitis, the true prevalence is unknown. In recent years, there have been several attempts to classify chronic pancreatitis, but these have met with difficulty for several reasons. -

The Role of Endoscopy in Ampullary and Duodenal Adenomas
GUIDELINE The role of endoscopy in ampullary and duodenal adenomas This is one of a series of statements discussing the use and mortality rates ranging from 1% to 9%,1,5-7 although of gastrointestinal endoscopy in common clinical situa- complication rates tend to be related to surgical case tions. The Standards of Practice Committee of the Amer- volume. ican Society for Gastrointestinal Endoscopy prepared this Endoscopic approaches for the evaluation and treat- text. In preparing this guideline, MEDLINE and PubMed ment of ampullary adenomas now represent a viable alter- databases were used to search publications through the native to surgical therapy. last 15 years related to ampullary and duodenal adeno- mas by using the keyword(s) ‘‘ampullary adenoma’’ and Evaluation of ampullary lesions before each of the following: ‘‘ampullectomy,’’ ‘‘duodenal endoscopic therapy adenoma,’’ and ‘‘familial adenomatous polyposis.’’ Ampullary adenomas cannot always be distinguished The search was supplemented by accessing the ‘‘related from ampullary carcinomas or nonadenomatous polyps articles’’ feature of PubMed with articles identified on (carcinoid tumors, gangliocytic paragangliomas, etc) on MEDLINE and PubMed as the references. Pertinent studies the basis of endoscopic appearance alone. Suspicious am- published in English were reviewed. Studies or reports pullary lesions should be biopsied before endoscopic re- that described fewer than 10 patients were excluded section is attempted. Brush cytology may offer additional from analysis if multiple series with greater than 10 information to biopsy for the detection of malignancy in patients addressing the same issue were available. selected cases.8 Recommendations were made on the basis of the re- There is no consensus on which ampullary adenomas viewed studies and were graded as to the strength of the should be kept under surveillance and which lesions ( ).