Pecatonica River Total Maximum Daily Load and Load Reduction Strategies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
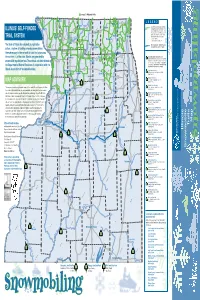
Illinois Snowmobile Trails
Connects To Wisconsin Trails East g g Dubuque g Warren L E G E N D 26 Richmond 173 78 Durand E State Grant Assisted Snowmobile Trails N Harvard O Galena O on private lands, open to the public. For B ILLINOIS’ SELF-FUNDED 75 E K detailed information on these trails, contact: A 173 L n 20 Capron n Illinois Association of Snowmobile Clubs, Inc. n P.O. Box 265 • Marseilles, IL 61341-0265 N O O P G e A McHenry Gurnee S c er B (815) 795-2021 • Fax (815) 795-6507 TRAIL SYSTEM Stockton N at iv E E onica R N H N Y e-mail: [email protected] P I R i i E W Woodstock N i T E S H website: www.ilsnowmobile.com C Freeport 20 M S S The State of Illinois has adopted, by legislative E Rockford Illinois Department of Natural Resources I 84 l V l A l D r Snowmobile Trails open to the public. e Belvidere JO v action, a system of funding whereby snowmobilers i R 90 k i i c Algonquin i themselves pay for the network of trails that criss-cross Ro 72 the northern 1/3 of the state. Monies are generated by Savanna Forreston Genoa 72 Illinois Department of Natural Resources 72 Snowmobile Trail Sites. See other side for detailed L L information on these trails. An advance call to the site 64 O Monroe snowmobile registration fees. These funds are administered by R 26 R E A L is recommended for trail conditions and suitability for C G O Center Elgin b b the Department of Natural Resources in cooperation with the snowmobile use. -

The Driftless Area – a Physiographic Setting (Dale K
A Look Back at Driftless Area Science to Plan for Resiliency in an Uncertain Future th Special Publication of the 11 Annual Driftless Area Symposium 1 A Look Back at Driftless Area Science to Plan for Resiliency in an Uncertain Future Special Publication of the 11th Annual Driftless Area Symposium Radisson Hotel, La Crosse, Wisconsin February 5th-6th, 2019 Table of Contents: Preface: A Look Back at Driftless Area Science to Plan for Resiliency in an Uncertain Future (Daniel C. Dauwalter, Jeff Hastings, Marty Melchior, and J. “Duke” Welter) ........................................... 1 The Driftless Area – A Physiographic Setting (Dale K. Splinter) .......................................................... 5 Driftless Area Land Cover and Land Use (Bruce Vondracek)................................................................ 8 Hydrology of the Driftless Area (Kenneth W. Potter) ........................................................................... 15 Geology and Geomorphology of the Driftless Area (Marty Melchior) .............................................. 20 Stream Habitat Needs for Brown Trout and Brook Trout in the Driftless Area (Douglas J. Dieterman and Matthew G. Mitro) ............................................................................................................ 29 Non-Game Species and Their Habitat Needs in the Driftless Area (Jeff Hastings and Bob Hay) .... 45 Climate Change, Recent Floods, and an Uncertain Future (Daniel C. Dauwalter and Matthew G. Mitro) ......................................................................................................................................................... -

EAST SIDE REVITALIZATION Reducing the Impacts of Flooding and Floodway Regulations Freeport, IL
DECEMBER 2013 EAST SIDE REVITALIZATION Reducing the Impacts of Flooding and Floodway Regulations Freeport, IL INTRODUCTION HOW DO FLOODING AND FLOODWAY REGULATIONS EPA’s Superfund Redevelopment Initiative (SRI) IMPACT THE EAST SIDE? and EPA Region 5 sponsored a reuse planning process for the CMC Heartland Site and other The East Side is an African-American neighborhood located in the floodway of the Pecatonica River. Residents of the East Side share a strong sense of community and contaminated properties in the East Side deep affection for the neighborhood. Many families have lived in the neighborhood neighborhood of Freeport, Illinois. The project for generations. Long-time residents remember a time when the neighborhood connects site reuse with area-wide neighborhood supported quality housing and thriving businesses with neighborhood-oriented revitalization for this environmental justice amenities. community. This report summarizes outcomes The neighborhood’s economic vitality and housing quality have been impacted from a 12-month community planning process, negatively over time by the neighborhood’s location in the floodway. Residents including considerations for reducing the impacts contend with recurring major and minor flood events, and are subject to Federal of flooding and floodway regulations on the East Emergency Management Agency (FEMA) and State of Illinois floodway regulations, Side Neighborhood. which limit improvements on structures located in a floodway. These regulations, which were not in place when the neighborhood was built, make it challenging to COMMUNITY GOALS improve and expand both housing and neighborhood businesses. Over time, housing Neighborhood stakeholders identified two quality has severely declined and most commercial businesses have vacated the neighborhood. -

Pecatonica River Rapid Watershed Assessment Document
PECATONICA RIVER WATERSHED (WI) HUC: 07090003 Wisconsin Illinois Rapid Watershed Assessment Pecatonica River Watershed Rapid watershed assessments provide initial estimates of where conservation investments would best address the concerns of landowners, conservation districts, and other community organizations and stakeholders. These assessments help landowners and local leaders set priorities and determine the best actions to achieve their goals. Wisconsin June 2008 The United States Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, and marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326W, Whitten Building, 14th and Independence Avenue, SW, Washington DC 20250-9410, or call (202) 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. PECATONICA RIVER WATERSHED (WI) HUC: 07090003 Contents INTRODUCTION 1 COMMON RESOURCE AREAS 3 ASSESSMENT OF WATERS 5 SOILS 7 DRAINAGE CLASSIFICATION 8 FARMLAND CLASSIFICATION 9 HYDRIC SOILS 10 LAND CAPABILITY CLASSIFICATION 11 RESOURCE CONCERNS 12 PRS PERFORMANCE MEASURES 12 CENSUS AND SOCIAL DATA (RELEVANT) 13 POPULATION ETHNICITY 14 URBAN POPULATION 14 ECOLOGICAL LANDSCAPES 15 WATERSHED ASSESSMENT 16 WATERSHED PROJECTS, STUDIES, MONITORING, ETC. 16 PARTNER GROUPS 17 FOOTNOTES/BIBLIOGRAPHY 18 PECATONICA RIVER WATERSHED (WI) HUC: 07090003 INTRODUCTION 1. The Pecatonica River watershed encompasses over 1.2 million acres southwest Wisconsin and northwest Illinois. -

Rock River Basin: Historical Background, Iepa Targeted Watersheds, and Resource-Rich Areas
ROCK RIVER BASIN: HISTORICAL BACKGROUND, IEPA TARGETED WATERSHEDS, AND RESOURCE-RICH AREAS by Robert A. Sinclair Office of Surface Water Resources: Systems, Information & GIS Illinois State Water Survey Hydrology Division Champaign, Illinois A Division of the Illinois Department of Natural Resources April 1996 CONTENTS Page Introduction 1 Acknowledgments 1 Historical Perspective of the Basin 3 Basin Physiography and Geology 3 Land Use 5 Basin Soils 5 Streams in the Basin 5 Streamflow and Sediment Quality 8 Streamflow 8 Sediment Quality 8 Surface Water Quality 13 IEPA Stream Assessment Criteria 13 IEPA Surface Water Quality Assessment 14 Ground-Water Resources and Quality 16 Ground-Water Resources 16 Ground-Water Quality 17 IEPA Targeted Watershed Approach 19 Major Watershed Areas 19 Criteria for Selecting Targeted Watersheds 19 TWA Program Activities 19 TWA for the Rock River Basin 20 References 23 INTRODUCTION Conservation 2000, a program to improve natural ecosystems, visualizes resource-rich areas, and promotes ecosystem projects and development of procedures to integrate economic and recreational development with natural resource stewardship. The Rock River basin is one of the areas identified for such projects. This report briefly characterizes the water resources of the basin and provides other relevant information. The Rock River originates in the Horicon Marsh in Dodge County, Wisconsin, and flows in a generally southerly direction until it enters Illinois just south of Beloit. Then it flows in a southwesterly direction until it joins the Mississippi River at Rock Island. The river flows for about 163 miles in Illinois, and its total length from head to mouth is about 318 miles. -

Chapter 2: Analysis of the Driftless Area
CHAPTER 2: ANALYSIS OF THE DRIFTLESS AREA. A. INTRODUCTION AND DEFINING THE OVERALL PLANNING AREA “The health of our waters is the principal measure of how we live on the land.” ‐Luna Leopold Although a sentiment that applies universally, in likely no part of the state is this passage truer than the Driftless Area.1 With its steep topography and deep deposits of wind‐blown loess soil, land use activities in the region have a direct and dramatic influence on the quality and quantity of the water in streams and rivers. The large‐scale changes to vegetation that began with Euro‐American settlement beginning in the early 1800s brought major transformations to the streams and rivers of western and southwestern Wisconsin. As ridge tops and hills were plowed and cows grazed the steep hillsides, more and more topsoil washed down slope. In some drainages, over ten feet of soil covered valley floors. Rivers and streams that had meandered around rocks and boulders on gravel and sand beds were converted into sediment‐laden waters incised in narrow, dirt‐lined, deep channels. Where once healthy populations of brook trout and other native species dependent on cool, clean water thrived, only green sunfish, mudminnow, golden shiner, and common carp were common. This condition began to improve in the 1930s as the impacts that poor farm management practices had on environmental health were increasingly recognized. Coon Valley in Vernon County became the nation’s first watershed conservation project – a landscape‐scale, multi‐disciplinary approach to improving both the productivity of farms and quality of rivers and streams. -

1. Green Cities
Illinois Wildlife Action Plan Update 1. Green Cities - Metropolitan Areas Campaign The Green Cities/Metropolitan Areas Campaign of the Illinois Wildlife Action Plan advances habitat conservation and restoration in support of wildlife species within the state's developed Metropolitan areas. Metropolitan areas have higher levels of human population density with greater levels of structural development. These areas, however, still sustain significant wildlife and, as a result, having a wildlife action plan dedicated to these areas is necessary to have a complete and effective wildlife management strategy. It is the goal of this campaign to look at the interaction of humans and natural resources to: provide recommendations to protect, maintain and enhance resource function and species survival in urban areas; and address urban development patterns to help establish resilient and vibrant Green Cities in Illinois. Illinois’ population is concentrated within a number of urban areas throughout Illinois. Urban areas were isolated first by using the US Census Bureau’s Metropolitan Statistical Areas (MSA)1 designation as primary basis for identification. A Metropolitan Statistical Area has an urban core with a population of 50,000 or more and it can contain multiple counties that either include the core area or are integrated socially and economically into the urban core. For purposes of this campaign, eight Illinois Metropolitan/Urban Focus Areas have been identified: Rockford Metropolitan Area; Chicago Metropolitan Area; Quad Cities Metropolitan Area; Peoria Metropolitan Area; Bloomington & Champaign/Urbana Metropolitan Area; Springfield/Decatur Metropolitan Area; East St. Louis Metropolitan Area; and Carbondale/Marion Metropolitan Area. The whole of the Metropolitan Planning Area for the Quad Cities, Rockford, Peoria, and East St. -

Pecatonica River Watershed TMDL Report THIS PAGE LEFT INTENTIONALLY BLANK
Bureau of Water Illinois P.O. Box 19276 Springfield, IL Environmental 62794-9276 Protection Agency www.epa.illinois.gov July 2018 IEPA/BOW/18-005 Pecatonica River Watershed TMDL Report THIS PAGE LEFT INTENTIONALLY BLANK olso erg), •-ss, L.) UNITED STATES ENVIRONMENTAL PROTECTION AGENCY g Za LT/ o (.7 REGION 5 z- i Tr so 2 77 WEST JACKSON BOULEVARD 44 PROT C' CHICAGO, IL 60604-3590 REPLY TO THE ATTENTION OF: JUL 2 5 MO WW-16J Sanjay Sofat, Chief Bureau of Water Illinois Environmental Protection Agency P.O. Box 19276 Springfield, Illinois 62794-9276 Dear Mr. Sofat: The U.S. Environmental Protection Agency has conducted a complete review of the final Total Maximum Daily Loads (TMDL) for fecal coliform and phosphorus for the Pecatonica River, including supporting documentation and follow up information. The waterbody is located in northwestern Illinois. The TMDLs submitted by the Illinois Environmental Protection Agency address the impaired Primary Contact and Aquatic Life Uses for the waterbodies. The TMDLs meet the requirements of Section 303(d) of the Clean Water Act and EPA's implementing regulations at 40 C.F.R. Part 130. Therefore, EPA hereby approves Illinois's six TMDLs for fecal coliform and phosphorus as noted in the enclosed decision document. The statutory and regulatory requirements, and EPA's review offilinois's compliance with each requirement, are described in the enclosed decision document. We wish to acknowledge Illinois's effort in submitting these TMDLs and look forward to future TMDL submissions by the State of Illinois. If you have any questions, please contact Mr. -
![Catfish Management Plan [PDF]](https://docslib.b-cdn.net/cover/8759/catfish-management-plan-pdf-2878759.webp)
Catfish Management Plan [PDF]
Distribution, Biology, and Management of Wisconsin’s Ictalurids Statewide catfish management plan April 2016 INTRODUCTION Wisconsin's Ictalurids can be classed into three broad groups. The bullheads – yellow, brown and black – are closely related members of the genus Ameiurus. The madtoms – slender, tadpole and stonecat – all belong to the genus Noturus. The two large catfish – the channel catfish and flathead catfish – are not closely related, but are linked by their importance as recreational and commercial species. Ictalurids are found in all three of Wisconsin's major drainages – the Mississippi River, Lake Michigan, and Lake Superior. Wisconsin has over 3,000 river miles of catfish water, along with several lakes and reservoirs that also support populations. They are most common in the Mississippi River and in the southern parts of the state. Although Wisconsin’s Ictalurids, such as channel catfish, do not enjoy the widespread glamour and status that they do in the southern United States, they are becoming more popular with Wisconsin’s anglers. A 2006-2007 mail survey revealed nearly 800,000 channel catfish were caught, while the harvest rate was nearly 70%. This harvest rate was highest among fish species targeted by Wisconsin anglers. Channel catfish are also an important commercial fishery on the Mississippi River, while setline anglers are active in many rivers of the state. As for flathead catfish, anecdotal evidence suggests that Wisconsin’s largest predatory fish is becoming a highly sought after trophy specimen. Given anglers’ increased propensity to fish for a food source as well as to fish for trophies, channel catfish and flathead catfish do not appear to have peaked in popularity. -

Selling Location: Illinois Town Advertisements, 1835-1837
SELLING LOCATION ILLINOIS TOWN ADVERTISEMENTS 1835-1837 William D. Walters, Jr. Professor Emeritus Department of Geography-Geology Illinois State University Normal, Illinois 2010 Copyright 2010 by William D. Walters, Jr. Published 2010 by the Department of Geography-Geology Illinois State University Normal IL 61790-4400 309-438-7649 Copies of this publication are available from the publisher. Price: $10.00 SELLING LOCATION ILLINOIS TOWN ADVERTISEMENTS 1835-1937 William D. Walters, Jr. This document consists of two parts. Part I is an introduction to Illinois towns and town selling during the boom of 1835-1837 when as many as one thousand new towns were laid out. Part II is a summary, in standard format, of over one hundred advertisements for new towns created during these years. These are followed by Appendix A, which lists the names of all proprietors named in the advertisements, and Appendix B, which is a list of all towns platted during this period in central Illinois. PART I INTRODUCTION TO TOWNS AND TOWN SELLING THE BOOM The town-founding boom of 1836 profoundly altered the American landscape, yet it is a frequently misunderstood event. Between 1835 and 1837 the human geography of Illinois was more strikingly altered than in any comparable period in the history of the state. The same is true of the surrounding states and of much of the Old South. Settlers poured in. Public land was sold in unprecedented amounts and an astonishing number of new towns were laid out. In much of central Illinois one third of all towns ever founded were platted in the few months between the spring of 1835 and the fall of 1837. -

Winnebago County Hydrologic and Hydraulic Analysis, Proposed Scope of Work
Winnebago County Hydrologic and Hydraulic Analysis, Proposed Scope of Work Illinois State Water Survey, Coordinated Hazard Assessment Program 1/5/2012 Winnebago County Hydrologic and Hydraulic Analysis, Proposed Scope of Work Contents Introduction .................................................................................................................................................. 2 Scope of Work ............................................................................................................................................... 3 South Ditch/Howard Creek ....................................................................................................................... 3 Spring Creek .............................................................................................................................................. 3 Fuller Creek ............................................................................................................................................... 4 Unnamed Tributary to Willow Creek ........................................................................................................ 4 Unnamed Tributary to McDonald Creek ................................................................................................... 5 Rock Fork South Drainage Ditch ............................................................................................................... 5 North Kent Creek ..................................................................................................................................... -

Illinois Bike Trails Map
Illinois Bike Trails Map 8 21 Grand Illinois Trail 56 65 16 12 49 4 52 61 Statewide Trails 66 Northeast 1. Burnham Greenway The Route 66 Trail combines sections of Historic Route 66, nearby roads, and off-road 36 2. Busse Woods Bicycle Trail 30 37 trails for bicyclists and other non-motorized travelers. From Chicago to St. Louis, over 400 26 3. Centennial Trail 63 27 miles are available along three historic road alignments. See www. bikelib. org/ maps- and - 62 64 18 4. Chain O’ Lakes State Park Trails 5. Chicago Lakefront Path rides/ route-guides/route-66-trail/ for route information. To learn about the Historic Route 66 Rockford 60 6. Danada-Herrick Lake Regional Trail Scenic Byway, visit www.illinoisroute 66. org. For more information, contact the Illinois De- 40 7. Des Plaines River Trail (Cook County) 39 90 33 15 partment of Natural Resources, 217/782-3715. 7 8. Des Plaines River Trail (Lake County) 9. DuPage River Trail 35 Mississippi 2 10. East Branch DuPage River Greenway Trail The Grand Illinois Trail is a 500-mile loop of off-road trails and on-road bicycle routes, River 290 11. Fox River Trail ILL Trail 94 31 D IN 11 12. Grant Woods Forest Preserve Trail N O joined together across northern Illinois, stretching from Lake Michigan to the Mississippi 190 A 29 I 13. Great Western Trail (Kane & DeKalb counties) R S River. Metropolitan areas, rural small towns, historic landmarks, and scenic landscapes and 13 39 28 G 51 14. Great Western Trail (DuPage County) 53 59 50 294 parks are woven together by the Grand Illinois Trail, offering a superb bicycling experience.