Catfish Management Plan [PDF]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

8 Va Tech Blue Catfish Sdafs Alosa Predation
Predation of Alosa Species by Non-native Catfish in Virginia’s Tidal Rivers Joseph D. Schmitt and Donald J. Orth Department of Fish and Wildlife Conservation Virginia Tech 100 Cheatham Hall, Blacksburg, VA 24061 Background What is the impact of blue and flathead catfish on Alosa species? • During the spring • Also, as juveniles migrate downriver during the fall • Data has been collected on James, York, and Rappahannock Background Declining American Shad and River Herring fisheries (ASMFC). Objectives 1.) Quantify predation of Alosa species during the spring 2.) Determine whether or not blue & flathead catfish are selectively feeding on Alosa species 3.) Determine if Alosa predation varies spatially Very limited information on the diet of fish > 600 mm particularly during the spring There are no published accounts of flathead catfish food habits in Virginia’s tidal rivers Methods - 20 km section below fall line on James River - Alosa species congregate here - Sampled March – May, as this corresponds with the Anadromous spawning run - Random sampling design - Rivers divided into 0.5 km sections - Random site selection - High-frequency electrofishing for most of the time (LF ineffective in temps < 18 C; Bodine and Shoup 2010) Catfish were also sampled in areas known to hold Alosines in the spring: -Bosher Dam -Belle Isle -Gordon Creek -Herring Creek -Ward Creek Catfish were also sampled in areas known to hold Alosines in the spring: -Bosher Dam -Belle Isle -Gordon Creek -Herring Creek -Ward Creek - Coordinates, tide phase, fish length, fish weight, temp recorded for each site/fish - Diet items extracted using pulsed gastric lavage (Waters et al. -

Channel Catfish Life History and Biology
SRAC Publication No. 180 Southern Regional Aquaculture Center December, 1988 . Channel Catfish Life History and Biology Thomas L. Wellborn* Channel cattish, Ictalurus punctatus Rocky Mountains. Since then chan- is located on the back between the (Rafinesque), is the most important nel catfish have been widely intro- dorsal and caudal fins (Fig. 1). One species of aquatic animal commer- duced throughout the United States conspicuous characteristic of all cially cultured in the United States. and the world. catfish is the presence of barbels It belongs to the family Ictaluridae, around the mouth. The barbels are order Siluriformes. Members of the Physical characteristics arranged in a definite pattern with order Siluriformes are found in fresh Like all native North American cat- four under the jaw and one on each and salt water worldwide. There are fishes, a channel catfish has a body tip of the maxilla (upper jaw). at least 39 species of catfish in North that is cylindrical in cross-section, America, but only six have been cul- and lacks scales. Fins are soft-rayed The channel catfish is the only tured or have potential for commer- except for the dorsal and pectoral spotted North American catfish with cial production. They are the blue fins which have sharp, hard spines a deeply forked tail. There are 24-29 catfish, Ictalurus furcatus (LeSueur); that can inflict a nasty, painful rays in the anal fin. They are general- the white catfish, Ictalurus catus wound if a catfish is handled care- ly olivaceous to blue on the back, (Linnaeus); the black bullhead, Ic- lessly. -

Kansas Fishing Regulations Summary
2 Kansas Fishing 0 Regulations 0 5 Summary The new Community Fisheries Assistance Program (CFAP) promises to increase opportunities for anglers to fish close to home. For detailed information, see Page 16. PURCHASE FISHING LICENSES AND VIEW WEEKLY FISHING REPORTS ONLINE AT THE DEPARTMENT OF WILDLIFE AND PARKS' WEBSITE, WWW.KDWP.STATE.KS.US TABLE OF CONTENTS Wildlife and Parks Offices, e-mail . Zebra Mussel, White Perch Alerts . State Record Fish . Lawful Fishing . Reservoirs, Lakes, and River Access . Are Fish Safe To Eat? . Definitions . Fish Identification . Urban Fishing, Trout, Fishing Clinics . License Information and Fees . Special Event Permits, Boats . FISH Access . Length and Creel Limits . Community Fisheries Assistance . Becoming An Outdoors-Woman (BOW) . Common Concerns, Missouri River Rules . Master Angler Award . State Park Fees . WILDLIFE & PARKS OFFICES KANSAS WILDLIFE & Maps and area brochures are available through offices listed on this page and from the PARKS COMMISSION department website, www.kdwp.state.ks.us. As a cabinet-level agency, the Kansas Office of the Secretary AREA & STATE PARK OFFICES Department of Wildlife and Parks is adminis- 1020 S Kansas Ave., Rm 200 tered by a secretary of Wildlife and Parks Topeka, KS 66612-1327.....(785) 296-2281 Cedar Bluff SP....................(785) 726-3212 and is advised by a seven-member Wildlife Cheney SP .........................(316) 542-3664 and Parks Commission. All positions are Pratt Operations Office Cheyenne Bottoms WA ......(620) 793-7730 appointed by the governor with the commis- 512 SE 25th Ave. Clinton SP ..........................(785) 842-8562 sioners serving staggered four-year terms. Pratt, KS 67124-8174 ........(620) 672-5911 Council Grove WA..............(620) 767-5900 Serving as a regulatory body for the depart- Crawford SP .......................(620) 362-3671 ment, the commission is a non-partisan Region 1 Office Cross Timbers SP ..............(620) 637-2213 board, made up of no more than four mem- 1426 Hwy 183 Alt., P.O. -

A Practical Handbook for Determining the Ages of Gulf of Mexico And
A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes THIRD EDITION GSMFC No. 300 NOVEMBER 2020 i Gulf States Marine Fisheries Commission Commissioners and Proxies ALABAMA Senator R.L. “Bret” Allain, II Chris Blankenship, Commissioner State Senator District 21 Alabama Department of Conservation Franklin, Louisiana and Natural Resources John Roussel Montgomery, Alabama Zachary, Louisiana Representative Chris Pringle Mobile, Alabama MISSISSIPPI Chris Nelson Joe Spraggins, Executive Director Bon Secour Fisheries, Inc. Mississippi Department of Marine Bon Secour, Alabama Resources Biloxi, Mississippi FLORIDA Read Hendon Eric Sutton, Executive Director USM/Gulf Coast Research Laboratory Florida Fish and Wildlife Ocean Springs, Mississippi Conservation Commission Tallahassee, Florida TEXAS Representative Jay Trumbull Carter Smith, Executive Director Tallahassee, Florida Texas Parks and Wildlife Department Austin, Texas LOUISIANA Doug Boyd Jack Montoucet, Secretary Boerne, Texas Louisiana Department of Wildlife and Fisheries Baton Rouge, Louisiana GSMFC Staff ASMFC Staff Mr. David M. Donaldson Mr. Bob Beal Executive Director Executive Director Mr. Steven J. VanderKooy Mr. Jeffrey Kipp IJF Program Coordinator Stock Assessment Scientist Ms. Debora McIntyre Dr. Kristen Anstead IJF Staff Assistant Fisheries Scientist ii A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes Third Edition Edited by Steve VanderKooy Jessica Carroll Scott Elzey Jessica Gilmore Jeffrey Kipp Gulf States Marine Fisheries Commission 2404 Government St Ocean Springs, MS 39564 and Atlantic States Marine Fisheries Commission 1050 N. Highland Street Suite 200 A-N Arlington, VA 22201 Publication Number 300 November 2020 A publication of the Gulf States Marine Fisheries Commission pursuant to National Oceanic and Atmospheric Administration Award Number NA15NMF4070076 and NA15NMF4720399. -

WISCONSIN DNR FISHERIES INFORMATION SHEET Walleye
WISCONSIN DNR FISHERIES INFORMATION SHEET LAKE MINNESUING, DOUGLAS COUNTY 2017 The WDNR conducted a fisheries assessment of Lake Minnesuing, Douglas County from April 5 to April 13, 2017. Lake Minnesuing is a 432 acre drainage lake and has a maximum depth of 43 feet. The fishery includes panfish, largemouth bass, smallmouth bass, northern pike, and walleye. Lake Minnesuing was estimated to contain 207 adult walleye or 0.5 fish per acre. Adult Walleye Length Frequency Distribution 12 10 8 Walleye 6 Total Captured 73 Number Avg. Length (in.) 19.8 4 Length Range (in.) 12-26 2 % >14" 97% 0 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Length (Inches) Northern Pike Length Frequency Distribution 14 12 10 8 Northern Pike 6 Number Total Captured 105 4 Avg. Length (in.) 20.4 2 Length Range (in.) 14-39 0 %>26" 10% 13 15 17 19 21 23 25 27 29 31 33 35 37 39 Length (Inches) Bluegill Length Frequency Distribution 180 160 140 Bluegill 120 Total Captured 465 100 80 Avg. Length (in.) 4.1 Number 60 Length Range (in.) 2-9 40 % >7" 20% 20 0 1 2 3 4 5 6 7 8 9 10 Length (Inches) Black Crappie Length Frequency Distribution 35 30 25 Black Crappie 20 Total Captured 84 Number 15 Avg. Length (in.) 5.5 10 Length Range (in.) 3-11 % >8" 27% 5 0 2 3 4 5 6 7 8 9 10 11 12 Length (Inches) Other Species Species observed during this survey but not inluded in the report were brown bullhead, central mudminnow, common shiner, creek chub, largemouth bass, pumpkinseed, rock bass, white sucker, yellow bullhead, and yellow perch. -

Sandies, Hybrids Hot Bites
Hunting Texas Special section inside * August 8, 2008 Texas’ Premier Outdoor Newspaper Volume 4, Issue 24 * Hunting Annual 2008 www.lonestaroutdoornews.com INSIDE HUNTING Sandies, hybrids hot bites Schools keep anglers in class The Texas Animal Health Commission approved new BY CRAIG NYHUS rules permitting the transport of male hogs to Summer means hot white bass and hybrid striped authorized game ranches bass action at many Texas lakes, and North Texas without requiring blood lakes like Lake Ray Hubbard, Ray Roberts, Lewisville tests for swine disease. and Richland Chambers lead the way for many. Page 6 Gary Goldsmith, a retired principal, fished Lewisville Lake with Art Kenney and Michael The U.S. Fish and Wildlife Anderson. “We caught and released more than 100 Service approved liberal sand bass reaching the 2-pound mark,” Goldsmith waterfowl limits for the said. “With 30 minutes of daylight left we went to an 2008-2009 season. area called Queen’s Point for hybrids. As soon as we Page 7 started the bite was on — we caught 20 more fish at that spot.” FISHING The group was fishing Lead Babies Slabs in 18 feet of water. “It’s best to keep them as close to the bottom as possible when fishing for hybrids,” Goldsmith said. East Texas lakes find crappie fishermen switching gears to chase sandies when the crappie bite slows. West Texas reservoirs see the whites hitting on top. And in the Hill Country, the Highland Lakes often get hot. “All of the fish are on the main lakes,” said Joe Bray, who guides on several Hill Country lakes. -

The Koi Herpesvirus (Khv): an Alloherpesviru
Aquacu nd ltu a r e s e J Bergmann et al., Fish Aquac J 2016, 7:2 i o r u e r h n http://dx.doi.org/10.4172/2150-3508.1000169 s a i l F Fisheries and Aquaculture Journal ISSN: 2150-3508 ResearchResearch Artilce Article OpenOpen Access Access Is There Any Species Specificity in Infections with Aquatic Animal Herpesviruses?–The Koi Herpesvirus (KHV): An Alloherpesvirus Model Sven M Bergmann1*, Michael Cieslak1, Dieter Fichtner1, Juliane Dabels2, Sean J Monaghan3, Qing Wang4, Weiwei Zeng4 and Jolanta Kempter5 1FLI Insel Riems, Südufer 10, 17493 Greifswald-Insel Riems, Germany 2University of Rostock, Aquaculture and Sea Ranching, Justus-von-Liebig-Weg 6, Rostock 18059, Germany 3Aquatic Vaccine Unit, Institute of Aquaculture, School of Natural Sciences, University of Stirling, Stirling, FK9 4LA, UK 4Pearl-River Fisheries Research Institute, Xo. 1 Xingyu Reoad, Liwan District, Guangzhou 510380, P. R. of China 5West Pomeranian Technical University, Aquaculture, K. Królewicza 4, 71-550, Szczecin, Poland Abstract Most diseases induced by herpesviruses are host-specific; however, exceptions exist within the family Alloherpesviridae. Most members of the Alloherpesviridae are detected in at least two different species, with and without clinical signs of a disease. In the current study the Koi herpesvirus (KHV) was used as a model member of the Alloherpesviridae and rainbow trout as a model salmonid host, which were infected with KHV by immersion. KHV was detected using direct methods (qPCR and semi-nested PCR) and indirect (enzyme-linked immunosorbant assay; ELISA, serum neutralization test; SNT). The non-koi herpesvirus disease (KHVD)-susceptible salmonid fish were demonstrated to transfer KHV to naïve carp at two different temperatures including a temperature most suitable for the salmonid (15°C) and cyprinid (20°C). -

United States National Museum Bulletin 282
Cl>lAat;i<,<:>';i^;}Oit3Cl <a f^.S^ iVi^ 5' i ''*«0£Mi»«33'**^ SMITHSONIAN INSTITUTION MUSEUM O F NATURAL HISTORY I NotUTus albater, new species, a female paratype, 63 mm. in standard length; UMMZ 102781, Missouri. (Courtesy Museum of Zoology, University of Michigan.) UNITED STATES NATIONAL MUSEUM BULLETIN 282 A Revision of the Catfish Genus Noturus Rafinesque^ With an Analysis of Higher Groups in the Ictaluridae WILLIAM RALPH TAYLOR Associate Curator, Division of Fishes SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 IV Publications of the United States National Museum The scientific publications of the United States National Museum include two series, Proceedings of the United States National Museum and United States National Museum Bulletin. In these series are published original articles and monographs dealing with the collections and work of the Museum and setting forth newly acquired facts in the fields of anthropology, biology, geology, history, and technology. Copies of each publication are distributed to libraries and scientific organizations and to specialists and others interested in the various subjects. The Proceedings, begun in 1878, are intended for the publication, in separate form, of shorter papers. These are gathered in volumes, octavo in size, with the publication date of each paper recorded in the table of contents of the volume. In the Bulletin series, the first of which was issued in 1875, appear longer, separate publications consisting of monographs (occasionally in several parts) and volumes in which are collected works on related subjects. Bulletins are either octavo or quarto in size, depending on the needs of the presentation. Since 1902, papers relating to the botanical collections of the Museum have been published in the Bulletin series under the heading Contributions from the United States National Herbarium. -

Fish Inventory at Stones River National Battlefield
Fish Inventory at Stones River National Battlefield Submitted to: Department of the Interior National Park Service Cumberland Piedmont Network By Dennis Mullen Professor of Biology Department of Biology Middle Tennessee State University Murfreesboro, TN 37132 September 2006 Striped Shiner (Luxilus chrysocephalus) – nuptial male From Lytle Creek at Fortress Rosecrans Photograph by D. Mullen Table of Contents List of Tables……………………………………………………………………….iii List of Figures………………………………………………………………………iv List of Appendices…………………………………………………………………..v Executive Summary…………………………………………………………………1 Introduction…………………………………………………………………...……..2 Methods……………………………………………………………………………...3 Results……………………………………………………………………………….7 Discussion………………………………………………………………………….10 Conclusions………………………………………………………………………...14 Literature Cited…………………………………………………………………….15 ii List of Tables Table1: Location and physical characteristics (during September 2006, and only for the riverine sites) of sample sites for the STRI fish inventory………………………………17 Table 2: Biotic Integrity classes used in assessing fish communities along with general descriptions of their attributes (Karr et al. 1986) ………………………………………18 Table 3: List of fishes potentially occurring in aquatic habitats in and around Stones River National Battlefield………………………………………………………………..19 Table 4: Fish species list (by site) of aquatic habitats at STRI (October 2004 – August 2006). MF = McFadden’s Ford, KP = King Pond, RB = Redoubt Brannan, UP = Unnamed Pond at Redoubt Brannan, LC = Lytle Creek at Fortress Rosecrans……...….22 Table 5: Fish Species Richness estimates for the 3 riverine reaches of STRI and a composite estimate for STRI as a whole…………………………………………………24 Table 6: Index of Biotic Integrity (IBI) scores for three stream reaches at Stones River National Battlefield during August 2005………………………………………………...25 Table 7: Temperature and water chemistry of four of the STRI sample sites for each sampling date…………………………………………………………………………….26 Table 8 : Total length estimates of specific habitat types at each riverine sample site. -

Invasive Catfish Management Strategy August 2020
Invasive Catfish Management Strategy August 2020 A team from the Virginia Department of Game and Inland Fisheries uses electrofishing to monitor invasive blue catfish in the James River in 2011. (Photo by Matt Rath/Chesapeake Bay Program) I. Introduction This management strategy portrays the outcomes of an interactive workshop (2020 Invasive Catfish Workshop) held by the Invasive Catfish Workgroup at the Virginia Commonwealth University (VCU) Rice Rivers Center in Charles City, Virginia on January 29-30, 2020. The workshop convened a diverse group of stakeholders to share the current scientific understanding and priority issues associated with invasive catfishes in Chesapeake Bay. The perspectives shared and insights gained from the workshop were used to develop practical, synergistic recommendations that will improve management and mitigate impacts of these species across jurisdictions within the watershed. Blue catfish (Ictalurus furcatus) and flathead catfish (Pylodictis olivaris) are native to the Ohio, Missouri, Mississippi, and Rio Grande river basins, and were introduced into the Virginia tributaries of Chesapeake Bay in the 1960s and 1970s to establish a recreational fishery. These non-native species have since spread, inhabiting nearly all major tributaries of the Bay watershed. Rapid range expansion and population growth, particularly of blue catfish, have led to increasing concerns about impacts on the ecology of the Chesapeake Bay ecosystem. 1 Chesapeake Bay Management Strategy Invasive Catfish Blue and flathead catfishes are long-lived species that can negatively impact native species in Chesapeake Bay through predation and resource competition. Blue catfish are generalist feeders that prey on a wide variety of species that are locally abundant, including those of economic importance and conservation concern, such as blue crabs, alosines, Atlantic menhaden, American eels, and bay anchovy. -

Redalyc.First Record of the Exotic Channel Catfish
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Spindler da Cruz, Sabrina; Evangelista Leal, Mateus; Lehmann Albornoz, Pablo César; Horst Schulz, Uwe First record of the exotic channel catfish Ictalurus punctatus (Rafinesque 1818) (Siluriformes: Ictaluridae) in the Rio dos Sinos basin, RS, Brazil Biota Neotropica, vol. 12, núm. 3, septiembre, 2012, pp. 1-4 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199124391005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotrop., vol. 12, no. 3 First record of the exotic channel catfish Ictalurus punctatus (Rafinesque 1818) (Siluriformes: Ictaluridae) in the Rio dos Sinos basin, RS, Brazil Sabrina Spindler da Cruz1, Mateus Evangelista Leal1, Pablo César Lehmann Albornoz2 & Uwe Horst Schulz1,3 1Laboratório de Ecologia de Peixes, Universidade do Vale do Rio dos Sinos – UNISINOS, Av. Unisinos, 950, Cristo Rei, CEP 93022-000, São Leopoldo, RS, Brasil 2Laboratório de Ictiologia, Universidade do Vale do Rio dos Sinos – UNISINOS, Av. Unisinos, 950, Cristo Rei, CEP 93022-000, São Leopoldo, RS, Brasil 3Corresponding author: Schulz Uwe Horst, e-mail: [email protected] CRUZ-SPINDLER, S., LEAL, M.E.; LEHMANN, P.A. & SCHULZ, U.H. First record of the exotic channel catfish Ictalurus punctatus (Rafinesque 1818) (Siluriformes: Ictaluridae) in the Rio dos Sinos basin, RS, Brazil. Biota Neotrop. 12(3): http://www.biotaneotropica.org.br/v12n3/en/abstract?article+bn01212032012 Abstract: The introduction of non-native species in inland waters is one of the main threats for aquatic biodiversity. -
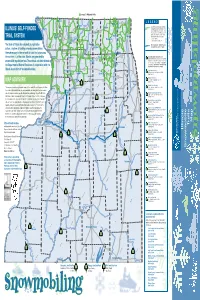
Illinois Snowmobile Trails
Connects To Wisconsin Trails East g g Dubuque g Warren L E G E N D 26 Richmond 173 78 Durand E State Grant Assisted Snowmobile Trails N Harvard O Galena O on private lands, open to the public. For B ILLINOIS’ SELF-FUNDED 75 E K detailed information on these trails, contact: A 173 L n 20 Capron n Illinois Association of Snowmobile Clubs, Inc. n P.O. Box 265 • Marseilles, IL 61341-0265 N O O P G e A McHenry Gurnee S c er B (815) 795-2021 • Fax (815) 795-6507 TRAIL SYSTEM Stockton N at iv E E onica R N H N Y e-mail: [email protected] P I R i i E W Woodstock N i T E S H website: www.ilsnowmobile.com C Freeport 20 M S S The State of Illinois has adopted, by legislative E Rockford Illinois Department of Natural Resources I 84 l V l A l D r Snowmobile Trails open to the public. e Belvidere JO v action, a system of funding whereby snowmobilers i R 90 k i i c Algonquin i themselves pay for the network of trails that criss-cross Ro 72 the northern 1/3 of the state. Monies are generated by Savanna Forreston Genoa 72 Illinois Department of Natural Resources 72 Snowmobile Trail Sites. See other side for detailed L L information on these trails. An advance call to the site 64 O Monroe snowmobile registration fees. These funds are administered by R 26 R E A L is recommended for trail conditions and suitability for C G O Center Elgin b b the Department of Natural Resources in cooperation with the snowmobile use.