Neuropathic Pain: Pregabalin and Gabapentin Prescribing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
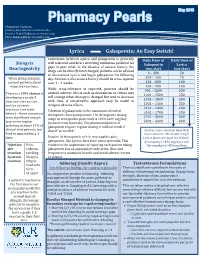
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas. -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management. -

Insomnia Disorder a VA Clinician’S Guide to Managing Insomnia Disorder (2019) Contents Insomnia Disorder
Insomnia Disorder A VA Clinician’s Guide to Managing Insomnia Disorder (2019) Contents Insomnia Disorder .................................................................................................... 3 Risks in elderly patients and patients with dementia .................................15 Background ................................................................................................................ 3 Provider perceptions vs reality ............................................................................16 Figure 1. Stepped Care for Management of Insomnia Disorder .............. 3 Figure 5. Weighing the potential risks versus benefits of Table 1. Brief summary of the ISI ......................................................................... 4 medication use .........................................................................................................16 Figure 2. Acute Insomnia to Insomnia Disorder ............................................ 5 Doxepin ........................................................................................................................17 Clinical Pearl ............................................................................................................... 5 Figure 6. Doxepin Use .............................................................................................18 Figure 3. Common causes of sleep disturbance ........................................... 6 Ramelteon ...................................................................................................................18 -

1 Gabapentinoids in Total Joint Arthroplasty: the Clinical Practice
1 Gabapentinoids in Total Joint Arthroplasty: The Clinical Practice Guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society Charles P. Hannon MD1, Yale A. Fillingham MD2, James A. Browne MD3, Emil H Schemitsch MD FRCS(C)4, AAHKS Anesthesia & Analgesia Clinical Practice Guideline Workgroup5, Asokumar Buvanendran MD6, William G. Hamilton MD7*, Craig J. Della Valle MD1* 1Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, IL, USA 2Department of Orthopaedic Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA 3Department of Orthopaedic Surgery, University of Virginia, Charlottesville, VA, USA 4Department of Surgery, University of Western Ontario, London, Ontario, Canada 5Workgroup Comprised of the following individuals: Justin T. Deen MD (Department of Orthopaedics and Rehabilitation, University of Florida College of Medicine, Gainesville, FL, USA), Greg A. Erens MD (Department of Orthopaedic Surgery, Emory University, Atlanta, GA, USA), Jess H. Lonner MD (Rothman Institute at Thomas Jefferson University, Philadelphia, PA, USA), Aidin E. Pour MD (Department of orthopaedic surgery, University of Michigan, Ann Arbor, MI, USA), Robert S. Sterling MD (Department of Orthopedic Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA) 6Department of Anesthesiology, Rush University Medical Center, Chicago, IL, USA 7Anderson Orthopedic Research Institute, Alexandria, VA, USA *Denotes co-senior authors 2 Introduction The American Association of Hip and Knee Surgeons (AAHKS), The American Academy of Orthopaedic Surgeons (AAOS), The Hip Society, The Knee Society and The American Society of Regional Anesthesia and Pain Medicine (ASRA) have worked together to develop evidence-based guidelines on the use of gabapentinoids in primary total joint arthroplasty (TJA). -

Gabapentin Enacarbil (Horizant) Monograph
Gabapentin enacarbil (Horizant) Monograph ® Gabapentin Enacarbil (Horizant ) National Drug Monograph March 2012 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. These documents will be updated when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. Executive Summary: Gabapentin enacarbil (Horizant®) is a prodrug of gabapentin which binds with high affinity to the α2δ subunit of voltage-activated calcium channels in vitro studies. It is unknown how the binding of gabapentin enacarbil (GEn) to the α2δ subunit corresponds to the treatment of restless leg syndrome (RLS) symptoms. Indication: GEn is FDA approved for treatment of RLS. Pharmacokinetics: GEn is primarily excreted by the kidneys and neither gabapentin nor GEn is substrate, inhibitor, or inducer of the major CYP450 system. In addition, GEn is not an inhibitor or substrate of p-glycoprotein in vitro. Dose: The recommended dosage is 600 mg once daily taken with food at about 5 PM. Efficacy: Several studies examining GEn have shown efficacy over placebo in patients with RLS; improvement in RLS symptoms was observed within 7 days of starting treatment.6-8, 12-14 Three studies demonstrated 1200 mg/day GEn significantly reduced International Restless Legs Syndrome (IRLS) scale total score and improved Clinical Global Impression– Improvement (CGI-I) scale compared with placebo.6-8 Two studies demonstrated efficacy at lower dose of 600 mg/day GEn; however, another study did not.7,12,13 These results are comparable to those seen with dopamine agonists.8,12 Adverse Drug Events: Commonly reported adverse effects for the approved dose of 600 mg/day GEn are somnolence, sedation and dizziness. -

Picquestion of the Week:2/16/09
Happy Presidents’ Day – The White House PIC QUESTION OF THE WEEK: 2/16/09 Q: Is there any basis for considering gabapentin a possible drug of abuse? A: Gabapentin (Neurontin, etc.) is an anticonvulsant structurally related to gamma-aminobutyric acid (GABA) and indicated for the treatment of postherpetic neuralgia and as an adjunct for partial seizures. It is chemically and structurally related to pregabalin (Lyrica), a Schedule V compound labeled for the treatment of fibromyalgia as well as diabetic and other forms of neuropathic pain. Gabapentin is not categorized as a scheduled substance; however, there is some question as to its potential for abuse. It has been used (off-label) for the management of a number of drug withdrawal syndromes including those related to the abuse of cocaine, alcohol, opioids, benzodiazepines, etc. Anticonvulsant medications enhance GABA activity and result in reduced cravings and the drug-seeking behavior associated with cocaine abuse. While these reports support the efficacy of gabapentin for reducing withdrawal symptoms from a variety of drugs of abuse, there is little information related to the actual misuse of this compound. One group of prison inmates with a history of intranasal cocaine dependence was reported to snort the powder from gabapentin capsules to experience a euphoric state similar to that provided by cocaine. While gabapentin was prescribed for labeled indications, the patients’ behaviors resulted in abuse of the drug. There are a number of additional cases of gabapentin abuse and withdrawal. In each of the reports, patients had a prior history of some type of substance abuse and were receiving daily doses ≥3,600 mg. -

Gabapentin Misuse, Abuse, and Diversion: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Addiction Manuscript Author . Author manuscript; Manuscript Author available in PMC 2017 August 29. Published in final edited form as: Addiction. 2016 July ; 111(7): 1160–1174. doi:10.1111/add.13324. Gabapentin misuse, abuse, and diversion: A systematic review Rachel V. Smith, MPH1,2,3, Jennifer R. Havens, PhD, MPH1,2, and Sharon L. Walsh, PhD1,4,5,* 1Center on Drug and Alcohol Research, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, KY 2Department of Epidemiology, University of Kentucky College of Public Health, Lexington, KY 3Department of Biostatistics, University of Kentucky College of Public Health, Lexington, KY 4Department of Pharmacology, University of Kentucky College of Medicine, Lexington, KY 5Department of Pharmaceutical Sciences, University of Kentucky College of Pharmacy, Lexington, KY Abstract Background and Aims—Since its market release, gabapentin has been presumed to have no abuse potential and subsequently has been prescribed widely off-label, despite increasing reports of gabapentin misuse. This review estimates and describes the prevalence and effects of, motivations behind, and risk factors for gabapentin misuse, abuse, and diversion. Methods—Databases were searched for peer-reviewed articles demonstrating gabapentin misuse, characterized by taking a larger dosage than prescribed or taking gabapentin without a prescription, and diversion. All types of studies were considered; grey literature was excluded. Thirty-three articles met inclusion criteria, consisting of 23 case studies and 11 epidemiological reports. Published reports came from the USA, the UK, Germany, Finland, India, South Africa, and France, and two analyzed websites not specific to a particular country. -

Treatment for Acute Pain: an Evidence Map Technical Brief Number 33
Technical Brief Number 33 R Treatment for Acute Pain: An Evidence Map Technical Brief Number 33 Treatment for Acute Pain: An Evidence Map Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. 290-2015-0000-81 Prepared by: Minnesota Evidence-based Practice Center Minneapolis, MN Investigators: Michelle Brasure, Ph.D., M.S.P.H., M.L.I.S. Victoria A. Nelson, M.Sc. Shellina Scheiner, PharmD, B.C.G.P. Mary L. Forte, Ph.D., D.C. Mary Butler, Ph.D., M.B.A. Sanket Nagarkar, D.D.S., M.P.H. Jayati Saha, Ph.D. Timothy J. Wilt, M.D., M.P.H. AHRQ Publication No. 19(20)-EHC022-EF October 2019 Key Messages Purpose of review The purpose of this evidence map is to provide a high-level overview of the current guidelines and systematic reviews on pharmacologic and nonpharmacologic treatments for acute pain. We map the evidence for several acute pain conditions including postoperative pain, dental pain, neck pain, back pain, renal colic, acute migraine, and sickle cell crisis. Improved understanding of the interventions studied for each of these acute pain conditions will provide insight on which topics are ready for comprehensive comparative effectiveness review. Key messages • Few systematic reviews provide a comprehensive rigorous assessment of all potential interventions, including nondrug interventions, to treat pain attributable to each acute pain condition. Acute pain conditions that may need a comprehensive systematic review or overview of systematic reviews include postoperative postdischarge pain, acute back pain, acute neck pain, renal colic, and acute migraine.