How to Provide Pain Relief for Laminitis in the Field
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Tramadol (Ultram)
TRAMADOL (ULTRAM) Tramadol is FDA approved for the treatment of musculoskeletal pain. Studies have shown it is useful in treating the pain associated with diabetic neuropathy and other pain conditions. Tramadol comes in 50 mg tablets. The maximum dose is two tablets four times per day unless your kidney function is below normal or you are over 75 years old, in which case the maximum dose is two tablets three times per day. The main side effects of Tramadol are drowsiness, sedation, and stomach upset, all of which are minimized by slowly raising the dose. About 5% of patients have stomach upset at any dose of Tramadol and cannot take the medicine. Other risks include seizures (occur in less than 1/100,000 and are more likely if you have seizures) and possibly abuse (relevant if you have abused drugs in the past). Tramadol should be started at a low dose and raise the dose slowly toward the maximum dose. Start with one tablet at bedtime. After 3 - 7 days, increase to one tablet twice daily (morning and bedtime). After an additional 3 - 7 days, increase to one tablet three times per day (morning, noon, and bedtime). After an additional 3 - 7 days, increase to one tablet four times per day (1 tablet with each meal and 1 at bedtime). At that point, the dose may be increased or adjusted depending on how you are doing. To increase further, you will: Add a second tablet at bedtime (one tablet three times per day and two tablets at bedtime). After 3 - 7 days, add a second tablet to another dose (one tablet twice per day and two tablets twice per day). -

Effects of Prophylactic Ketamine and Pethidine to Control Postanesthetic Shivering: a Comparative Study
Biomedical Research and Therapy, 5(12):2898-2903 Original Research Effects of prophylactic ketamine and pethidine to control postanesthetic shivering: A comparative study Masoum Khoshfetrat1, Ali Rosom Jalali2, Gholamreza Komeili3, Aliakbar Keykha4;∗ ABSTRACT Background: Shivering is an undesirable complication following general anesthesia and spinal anesthesia, whose early control can reduce postoperative metabolic and respiratory complications. Therefore, this study aims to compare the effects of prophylactic injection of ketamine and pethi- dine on postoperative shivering.Methods: This double-blind clinical trial was performed on 105 patients with short-term orthopedic and ENT surgery. The patients were randomly divided into three groups; 20 minutes before the end of the surgery, 0.4 mg/kg of pethidine was injected to the first group, 0.5 mg/kg of ketamine was injected to the second group, and normal saline was injected to the third group. After the surgery, the tympanic membrane temperature was measured at 0, 10, 20, and 30 minutes. The shivering was also measured by a four-point grading from zero (no shiv- ering) to four (severe shivering). Data were analyzed by one-way ANOVA, Kruskal Wallis, Chi-square 1Doctor of Medicine (MD), Fellow of and Pearson correlation. Results: The mean age of patients was 35.811.45 years in the ketamine Critical Care Medicine (FCCM), group, 34.811.64 years in the normal saline group, and 33.1110.5 years in the pethidine group. Department of Anesthesiology and The one-way ANOVA showed no significant difference in the mean age between the three groups Critical Care, Khatam-Al-Anbiya (P=0.645). -

Current Awareness in Clinical Toxicology Editors: Damian Ballam Msc and Allister Vale MD
Current Awareness in Clinical Toxicology Editors: Damian Ballam MSc and Allister Vale MD February 2016 CONTENTS General Toxicology 9 Metals 38 Management 21 Pesticides 41 Drugs 23 Chemical Warfare 42 Chemical Incidents & 32 Plants 43 Pollution Chemicals 33 Animals 43 CURRENT AWARENESS PAPERS OF THE MONTH How toxic is ibogaine? Litjens RPW, Brunt TM. Clin Toxicol 2016; online early: doi: 10.3109/15563650.2016.1138226: Context Ibogaine is a psychoactive indole alkaloid found in the African rainforest shrub Tabernanthe Iboga. It is unlicensed but used in the treatment of drug and alcohol addiction. However, reports of ibogaine's toxicity are cause for concern. Objectives To review ibogaine's pharmacokinetics and pharmacodynamics, mechanisms of action and reported toxicity. Methods A search of the literature available on PubMed was done, using the keywords "ibogaine" and "noribogaine". The search criteria were "mechanism of action", "pharmacokinetics", "pharmacodynamics", "neurotransmitters", "toxicology", "toxicity", "cardiac", "neurotoxic", "human data", "animal data", "addiction", "anti-addictive", "withdrawal", "death" and "fatalities". The searches identified 382 unique references, of which 156 involved human data. Further research revealed 14 detailed toxicological case reports. Current Awareness in Clinical Toxicology is produced monthly for the American Academy of Clinical Toxicology by the Birmingham Unit of the UK National Poisons Information Service, with contributions from the Cardiff, Edinburgh, and Newcastle Units. The NPIS is commissioned by Public Health England Current Awareness in Clinical Toxicology Editors: Damian Ballam MSc and Allister Vale MD February 2016 Current Awareness in Clinical Toxicology is produced monthly for the American Academy of Clinical Toxicology by the Birmingham Unit of the UK National Poisons Information Service, with contributions from the Cardiff, Edinburgh, and Newcastle Units. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Quantitative Drug Test Menu Section 2
1 Guthrie Square, Sayre, PA 18840 Bill To: Client GMG Toxicology Laboratory Requisition Toll Free Phone (844) 617-4719 Insurance Request Date: _____/______/______ Medical Director: Hani Hojjati, MD Fax (570) 887-4729 Patient PATIENT INFORMATION (PLEASE PRINT IN BLACK INK) INSURANCE BILLING INFORMATION (PLEASE PRINT IN BLACK INK) Pt Last Name First M I PRIMARY Medicare Medicaid Other Ins. Self Spouse Child __ Subscriber Last Name First M Address Birth Date Sex M F Beneficiary/Member # Group # City Pt. SS# or MRN Claims Name and Address City ST ZIP ST ZIP Home Phone (Attach a copy of the patient's insurance card and information) SECONDARY Medicare Medicaid Other Ins. Self Spouse Child Employer Work Phone Subscriber Last Name First M Work Address City ST ZIP Beneficiary/Member # Group # __ CLIENT INFORMATION - REFERRING PHYSICIAN Claims Name and Address City ST ZIP Client Address: (Atttach a copy of the patient's insurance card and information) COLLECTION / REPORTING INFORMATION Copy to: FAX Results to __ CALL Results to Phone: Fax: Date Collected: Time Collected: AM PM Specimen Type: Urine Saliva Other ___________________ Physician Signature (legible - No Stamp) For Lab Use Only (Required for Medicare & Medicaid patient orders) Signed ABN Obtained Place Lab Label Here Contact Laboratory Medical Director (570-887-4719) with questions concerning medical necessity PHYSICIAN When ordering tests, the physician is required to make an independent medical necessity decision with regard to each test thelaboratory will bill. The physician also understands he or she is required NOTICE to (1) submit ICD-10 diagnosis supported in the patient's medical record as documentation of the medical necessity or (2) explain and have the patient sign an ABN. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
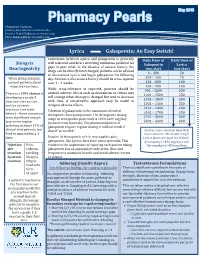
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas. -

CAN YOU TAKE TRAMADOL with NEFOPAM Can You Take Tramadol with Nefopam
CAN YOU TAKE TRAMADOL WITH NEFOPAM can you take tramadol with nefopam tramadol 37 5 vs percocet 5 325 ultram tramadol pictures tramadol hcl tabs 50 mg tramadol 200 mg recreational drugs and heart can tramadol and percocet be mixed hbs robaxin tramadol interaction generic tramadol 319 immediate release how long tramadol stay in your urine does tramadol make you sleepy or awake tramadol acetaminophen\/codeine 120 12mg sol b tracert ex tramadol dosage for adults meloxicam/tramadol/amitriptyline/lidocaine/prilocaine apo tramadol high feeling on hydrocodone tramadol apteka internetowa olmed order tramadol/paracetamol from mexico tramadol quizlet flashcards microbiology tramadol has mu opioid agonist activity director jobs tramadol met ritalin sr strengths hur ta tramadol withdrawal in dogs tramadol te gebruiken bij tramadol dosis cachorros bulldog 2015 100mg tramadol 10mg hydrocodone images 100 tramadol termasuk jenis obat apa acyclovir side how to get rid of a tramadol high 200 ml tramadol withdrawal timeline drug interactions between percocet and tramadol comparison tramadol e morfina presentacion de tres can tramadol be taken with paracetamol indication and action tramadol review article template with photos tramadol codeine allergy rash best price tramadol online tramadol 93 58 dosage for ibuprofen tramadol v oxycodone pill colors can tramadol make you drowsy doll b tracert ex tramadol addiction withdrawal tramadol instant release oxycontin pictures can you drink wine with tramadol i can function tramadol hydrochloride sleepy tramadol cva -

Parkinson's Disease Fact Sheet
Parkinson’s Disease Fact Sheet About Parkinson’s Disease Parkinson’s disease is a progressive, incurable neurological disorder associated with a loss of dopamine-generating cells in the brain. It is primarily associated with progressive loss of motor control, but it results in a complex array of symptoms, including many non-motor symptoms. Parkinson’s impacts an estimated one million people in the United States. Critical Clinical Care Considerations • To avoid serious side effects, Parkinson’s patients need their medications on time, every time — do not skip or postpone doses. • Write down the exact times of day medications are to be administered so that doses are given on the same schedule the patient follows at home. • Do not substitute Parkinson’s medications or stop levodopa therapy abruptly. • Resume medications immediately following procedures, unless vomiting or severely incapacitated. • If an antipsychotic is necessary, use pimavanserin (Nuplazid), quetiapine (Seroquel) or clozapine (Clozaril). • Be alert for symptoms of dysphagia (trouble swallowing) and risk of pneumonia. • Ambulate as soon as medically safe. Patients may require assistance. Common Symptoms of Parkinson’s Disease Motor Non-Motor • Shaking or tremor at rest • Depression • Bradykinesia or freezing (being stuck • Anxiety in place when attempting to walk) • Constipation • Low voice volume or muffled speech • Cognitive decline and dementia • Lack of facial expression • Impulse control disorders • Stiffness or rigidity of the arms, legs • Orthostatic hypotension or -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management.