Outpatient Treatment for Withdrawal from Alcohol and Benzodiazepines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Status Epilepticus
Published online: 2019-11-21 THIEME Review Article 267 Management of Status Epilepticus Ritesh Lamsal1 Navindra R. Bista1 1Department of Anaesthesiology, Tribhuvan University Teaching Address for correspondence Ritesh Lamsal, MD, DM, Department Hospital, Institute of Medicine, Tribhuvan University, Nepal of Anaesthesiology, Tribhuvan University Teaching Hospital, Institute of Medicine, Tribhuvan University,Kathmandu, Nepal (e-mail: [email protected]). J Neuroanaesthesiol Crit Care 2019;6:267–274 Abstract Status epilepticus (SE) is a life-threatening neurologic condition that requires imme- diate assessment and intervention. Over the past few decades, the duration of seizure Keywords required to define status epilepticus has shortened, reflecting the need to start thera- ► convulsive status py without the slightest delay. The focus of this review is on the management of con- epilepticus vulsive and nonconvulsive status epilepticus in critically ill patients. Initial treatment ► neurocritical care of both forms of status epilepticus includes immediate assessment and stabilization, ► nonconvulsive status and administration of rapidly acting benzodiazepine therapy followed by nonbenzodi- epilepticus azepine antiepileptic drug. Refractory and super-refractory status epilepticus (RSE and ► refractory status SRSE) pose a lot of therapeutic problems, necessitating the administration of contin- epilepticus uous infusion of high doses of anesthetic agents, and carry a high risk of debilitating ► status epilepticus morbidity as well as mortality. ► super-refractory sta- tus epilepticus Introduction occur after 30 minutes of seizure activity. However, this working definition did not indicate the need to immediately Status epilepticus (SE) is a medical and neurologic emergency commence treatment and that permanent neuronal injury that requires immediate evaluation and treatment. It is associat- could occur by the time a clinical diagnosis of SE was made. -

Insomnia Across the Lifespan: Treating This Common Condition
2/20/2019 Insomnia Across the Lifespan: Treating This Common Condition Wendy L. Wright MS, ANP-BC, FNP-BC, FAANP, FAAN, FNAP Adult / Family Nurse Practitioner Owner – Wright & Associates Family Healthcare @ Amherst and @ Concord, NH Owner – Partners in Healthcare Education © Wright, 2019 1 1 Disclosures • Speaker Bureau: Pfizer, Merck, Sanofi Pasteur • Consultant: Pfizer, Merck, Sanofi Pasteur © Wright, 2019 2 2 Objectives • Upon completion of this session, the participant will be able to: • Discuss the incidence and prevalence of insomnia across the lifespan • Identify the appropriate work-up of the individual with insomnia • Discuss nonpharmacologic and pharmacologic treatment options for the patient with insomnia © Wright, 2019 3 3 Wright, 2019 1 2/20/2019 What Is Insomnia? • Insomnia: • Difficulty initiating or maintaining sleep; sleep that is nonrestorative despite having an adequate opportunity and no abnormal environmental circumstances; and accompanied by daytime somnolence (Sateia, M.J. et. al, 2017) © Wright, 2019 4 4 What Is Insomnia? • DSM-V definition: • Difficulty initiating and / or • Difficulty maintaining and / or • Waking earlier than desired AND • Occurring at least 3 nights per week for at least 3 months AND • Dissatisfaction with sleep http://www.psychiatrictimes.com/special-reports/review-changes-dsm-5-sleep-wake-disorders accessed 08- 08-2018 © Wright, 2019 5 5 DSM-5 Diagnostic Criteria https://practicingclinicians.com/CE-CME/sleep/enhancing-the-management-of-insomnia-in-older-patients/MPCE90716 © Wright, 2019 6 -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Benzodiazepines: Uses and Risks Charlie Reznikoff, MD Hennepin Healthcare
Benzodiazepines: Uses and Risks Charlie Reznikoff, MD Hennepin healthcare 4/22/2020 Overview benzodiazepines • Examples of benzos and benzo like drugs • Indications for benzos • Pharmacology of benzos • Side effects and contraindications • Benzo withdrawal • Benzo tapers 12/06/2018 Sedative/Hypnotics • Benzodiazepines • Alcohol • Z-drugs (Benzo-like sleeping aids) • Barbiturates • GHB • Propofol • Some inhalants • Gabapentin? Pregabalin? 12/06/2018 Examples of benzodiazepines • Midazolam (Versed) • Triazolam (Halcion) • Alprazolam (Xanax) • Lorazepam (Ativan) • Temazepam (Restoril) • Oxazepam (Serax) • Clonazepam (Klonopin) • Diazepam (Valium) • Chlordiazepoxide (Librium) 4/22/2020 Sedatives: gaba stimulating drugs have incomplete “cross tolerance” 12/06/2018 Effects from sedative (Benzo) use • Euphoria/bliss • Suppresses seizures • Amnesia • Muscle relaxation • Clumsiness, visio-spatial impairment • Sleep inducing • Respiratory suppression • Anxiolysis/disinhibition 12/06/2018 Tolerance to benzo effects? • Effects quickly diminish with repeated use (weeks) • Euphoria/bliss • Suppresses seizures • Effects incompletely diminish with repeated use • Amnesia • Muscle relaxation • Clumsiness, visio-spatial impairment • Seep inducing • Durable effects with repeated use • Respiratory suppression • Anxiolysis/disinhibition 12/06/2018 If you understand this pharmacology you can figure out the rest... • Potency • 1 mg diazepam <<< 1 mg alprazolam • Duration of action • Half life differences • Onset of action • Euphoria, clinical utility in acute -

Drugs That Act in the Cns
DRUGS THAT ACT IN THE CNS Anxiolytic and Hypnotic Drugs Dr Karamallah S. Mahmood PhD Clinical Pharmacology 1 OTHER ANXIOLYTIC AGENTS/ A. Antidepressants Many antidepressants are effective in the treatment of chronic anxiety disorders and should be considered as first-line agents, especially in patients with concerns for addiction or dependence. Selective serotonin reuptake inhibitors (SSRIs) or serotonin/norepinephrine reuptake inhibitors (SNRIs) may be used alone or prescribed in combination with a benzodiazepine. SSRIs and SNRIs have a lower potential for physical dependence than the benzodiazepines and have become first-line treatment for GAD. 2 OTHER ANXIOLYTIC AGENTS/ B. Buspirone Buspirone is useful for the chronic treatment of GAD and has an efficacy comparable to that of the benzodiazepines. It has a slow onset of action and is not effective for short-term or “as-needed” treatment of acute anxiety states. The actions of buspirone appear to be mediated by serotonin (5-HT1A) receptors, although it also displays some affinity for D2 dopamine receptors and 5-HT2A serotonin receptors. Buspirone lacks the anticonvulsant and muscle-relaxant properties of the benzodiazepines. 3 OTHER ANXIOLYTIC AGENTS B. Buspirone The frequency of adverse effects is low, with the most common effects being headaches, dizziness, nervousness, nausea, and light-headedness. Sedation and psychomotor and cognitive dysfunction are minimal, and dependence is unlikely. Buspirone does not potentiate the CNS depression of alcohol. 4 V. BARBITURATES The barbiturates were formerly the mainstay of treatment to sedate patients or to induce and maintain sleep. Today, they have been largely replaced by the benzodiazepines, primarily because barbiturates induce tolerance and physical dependence and are associated with very severe withdrawal symptoms. -

DESCRIPTION CLINICAL PHARMACOLOGY Mechanism Of
NDA 20-844/ Topamav Sprinkle Capsules Approved Labeling Text Version: 10/26/98 DESCRIPTION Topiramate is a sulfamate-substituted monosaccharide that is intended for use as an antiepileptic drug. TOPAMAX@ (topiramate capsules) Sprinkle Capsules are available as I5 mg, 25 mg and 50mg sprinkle capsules for oral administration as whole capsules or for opening and sprinkling onto soft food. Topiramate is a white crystalline powder with a bitter taste. Topiramate is most soluble in alkaline solutions containing sodium hydroxide or sodium phosphate and hawng a pH of 9 to IO. It is freely soluble in acetone, chloroform, dimethylsulfoxide, and ethanol. The solubility in water is 9.8 mg/mL. Its saturated solution has a pH of 6.3. Topiramate has the molecular formula C,,H,,NO,S and a molecular weight of 339.37. Topiramate is designated chemically as 2,3:4,5-Di-O-isopropylidene-~- D-fructopyranose sulfamate and has the following structural formula: H3C CH3 TOPAMAX” (topiramate capsules) Sprinkle Capsules contain topiramate coated beads in a hard gelatin capsule. The inactive ingredients are: sugar spheres (sucrose and starch), povidone, cellulose acetate, gelatin, silicone dioxide, sodium lauryl sulfate, titanium dioxide, and black pharmaceutical ink. CLINICAL PHARMACOLOGY Mechanism of Action: The precise mechanism by which topiramate exerts its antiseizure effect is unknown; however, electrophysiological and biochemical studies of the effects of topiramate on cultured neurons have revealed three properties that may contribute to topiramate’s antiepileptic efficacy. First, action potentials elicited repetitively by a sustained depolarization of the neurons are blocked by topiramate in a time-dependent manner, suggestive of a state-dependent sodium channel blocking action. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Behandling Av Ångestsyndrom
Behandling av ångestsyndrom En systematisk litteraturöversikt Volym 2 September 2005 SBU • Statens beredning för medicinsk utvärdering The Swedish Council on Technology Assessment in Health Care SBU utvärderar sjukvårdens metoder SBU (Statens beredning för medicinsk utvärdering) är en statlig myn- Behandling av ångestsyndrom dighet som utvärderar sjukvårdens metoder. SBU analyserar nytta och kostnader för olika medicinska metoder och jämför vetenskapens stånd- punkt med svensk vårdpraxis. Målet är ett bättre beslutsunderlag för En systematisk litteraturöversikt alla som avgör vilken sjukvård som ska bedrivas. Välkommen att besöka SBU:s hemsida, www.sbu.se Volym 2 SBU ger ut tre serier av rapporter. I den första serien presenteras utvär- deringar som utförts av SBU:s projektgrupper. Dessa utvärderingar Projektgrupp åtföljs alltid av en sammanfattning och slutsatser fastställda av SBU:s Lars von Knorring Ingrid Håkanson styrelse och råd. Denna rapportserie ges ut med gula omslag. I den andra (ordförande) (projektassistent) serien, med vita omslag, presenteras aktuella kunskaper inom något Viveka Alton Lundberg Agneta Pettersson område av sjukvården där behov av utvärdering kan föreligga. Den tredje Vanna Beckman (projektledare under serien, Alert-rapporterna, avser tidiga bedömningar av nya metoder inom (deltog 1995–2002) perioden 2004–2005) hälso- och sjukvården. Susanne Bejerot Per-Anders Rydelius Roland Berg Sten Thelander (deltog 1995–2002) (projektledare under Cecilia Björkelund perioden 1995–2004) Per Carlsson Helene Törnqvist (deltog 1995–1999) (deltog 1999–2005) Elias Eriksson Kristian Wahlbeck (deltog 1995–2001) (deltog 2002–2005) Tom Fahlén Hans Ågren Mats Fredrikson Rapporten ”Behandling av ångestsyndrom” består av två volymer (nr 171/1+2) och kan beställas från: Externa granskare SBU, Box 5650, 114 86 Stockholm Fredrik Almqvist Per Høglend Besöksadress: Tyrgatan 7 Alv A. -
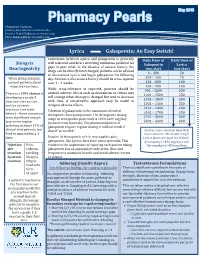
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Acute Migraine Treatment
Acute Migraine Treatment Morris Levin, MD Professor of Neurology Director, Headache Center UCSF Department of Neurology San Francisco, CA Mo Levin Disclosures Consulting Royalties Allergan Oxford University Press Supernus Anadem Press Amgen Castle Connolly Med. Publishing Lilly Wiley Blackwell Mo Levin Disclosures Off label uses of medication DHE Antiemetics Zolmitriptan Learning Objectives At the end of the program attendees will be able to 1. List all important options in the acute treatment of migraine 2. Discuss the evidence and guidelines supporting the major migraine acute treatment options 3. Describe potential adverse effects and medication- medication interactions in acute migraine pharmacological treatment Case 27 y/o woman has suffered ever since she can remember from “sick headaches” . Pain is frontal, increases over time and is generally accompanied by nausea and vomiting. She feels depressed. The headache lasts the rest of the day but after sleeping through the night she awakens asymptomatic 1. Diagnosis 2. Severe Headache relief Diagnosis: What do we need to beware of? • Misdiagnosis of primary headache • Secondary causes of headache Red Flags in HA New (recent onset or change in pattern) Effort or Positional Later onset than usual (middle age or later) Meningismus, Febrile AIDS, Cancer or other known Systemic illness - Neurological or psych symptoms or signs Basic principles of Acute Therapy of Headaches • Diagnose properly, including comorbid conditions • Stratify therapy rather than treat in steps • Treat early -

Accurate Measurement, and Validation of Solubility Data † ‡ ‡ § † ‡ Víctor R
Article Cite This: Cryst. Growth Des. 2019, 19, 4101−4108 pubs.acs.org/crystal In the Context of Polymorphism: Accurate Measurement, and Validation of Solubility Data † ‡ ‡ § † ‡ Víctor R. Vazqueź Marrero, , Carmen Piñero Berríos, , Luz De Dios Rodríguez, , ‡ ∥ ‡ § Torsten Stelzer,*, , and Vilmalí Lopez-Mej́ ías*, , † Department of Biology, University of Puerto RicoRío Piedras Campus, San Juan, Puerto Rico 00931, United States ‡ Crystallization Design Institute, Molecular Sciences Research Center, University of Puerto Rico, San Juan, Puerto Rico 00926, United States § Department of Chemistry, University of Puerto RicoRío Piedras Campus, San Juan, Puerto Rico 00931, United States ∥ Department of Pharmaceutical Sciences, University of Puerto RicoMedical Sciences Campus, San Juan, Puerto Rico 00936, United States *S Supporting Information ABSTRACT: Solubility measurements for polymorphic com- pounds are often accompanied by solvent-mediated phase transformations. In this study, solubility measurements from undersaturated solutions are employed to investigate the solubility of the two most stable polymorphs of flufenamic acid (FFA forms I and III), tolfenamic acid (TA forms I and II), and the only known form of niflumic acid (NA). The solubility was measured from 278.15 to 333.15 K in four alcohols of a homologous series (methanol, ethanol, 1- propanol, n-butanol) using the polythermal method. It was established that the solubility of these compounds increases with increasing temperature. The solubility curves of FFA forms I and III intersect at ∼315.15 K (42 °C) in all four solvents, which represents the transition temperature of the enantiotropic pair. In the case of TA, the solubility of form II could not be reliably obtained in any of the solvents because of the fast solvent- mediated phase transformation.