The Hyperbenthic Community Composition of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

SOSF 2020 Annual Report
SAVE OUR SEAS FOUNDATION ANNUAL REPORT 2020 SAVE OUR SEAS FOUNDATION ANNUAL REPORT 2020 “AS LONG AS THERE ARE PEOPLE WHO CARE AND TAKE ACTION, WE CAN AND WILL MAKE A DIFFERENCE.” THE FOUNDER | SAVE OUR SEAS FOUNDATION An oceanic manta ray in the Revil- lagigedo Archipelago National Park, Mexico. CONTENTS 02 FOUNDER’S STATEMENT 07 CEO’S FOREWORD 12 17 YEARS OF THE SAVE OUR SEAS FOUNDATION 14 WHERE WE WORK 16 OUR CENTRES 18 D’Arros Research Centre 24 Shark Education Centre 36 Shark Research Center 46 OUR PARTNERS 48 Bimini Biological Field Station Foundation 58 The Manta Trust 68 Shark Spotters 78 The North Coast Cetacean Society 88 The Acoustic Tracking Array Platform 98 PROJECT LEADERS 100 Small grant projects 110 Keystone projects | Continuation 118 Keystone projects | New 122 COMMUNICATION PROJECTS 132 OUR TEAM 140 FUNDING SUMMARY 140 Centres, partners and sponsorships 141 Index A: all projects funded in 2020 in alphabetical order 142 Index B: all projects funded in 2020 by type 144 Credits A group of whitetip reef sharks resting together on a ledge. CEO’S FOREWORD This year has not been the one any of us expected. As I write to reflect on 2020 amidst another lockdown, it is very hard to imagine a time before the current pandemic brought the world to a standstill. It has been a difficult period, with unparalleled loss for many. But there is hope. Vaccines are rolling out and we continue to adapt. Humanity is resourceful, and it has been humbling to witness the tremendous goodwill and sense of community as people work together to make this better. -

Population Productivity of Shovelnose Rays: Inferring the Potential for Recovery
bioRxiv preprint doi: https://doi.org/10.1101/584557; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Population productivity of shovelnose rays: inferring the potential for recovery 2 3 Short title: Population productivity of shovelnose rays 4 5 Brooke M. D’Alberto1, 2*, John K. Carlson3, Sebastián A. Pardo4, Colin A. Simpfendorfer1 6 7 1 Centre for Sustainable Tropical Fisheries and Aquaculture & College of Science and Engineering, 8 James Cook University, Townsville, Queensland, Australia 9 2 CSIRO Oceans and Atmosphere, Hobart, Tasmania, Australia. 10 3 NOAA/National Marine Fisheries Service–Southeast Fisheries Science Center, Panama City, FL. 11 4 Biology Department, Dalhousie University, Halifax, NS, Canada 12 13 *Corresponding author 14 Email: [email protected] (BMD) 15 16 17 18 19 20 21 22 23 24 25 bioRxiv preprint doi: https://doi.org/10.1101/584557; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 26 Abstract 27 Recent evidence of widespread and rapid declines of shovelnose ray populations (Order 28 Rhinopristiformes), driven by a high demand for their fins in Asian markets and the quality of their 29 flesh, raises concern about their risk of over-exploitation and extinction. -

Rhinobatos Punctifer, Spotted Guitarfish
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T161447A109904426 Scope: Global Language: English Rhinobatos punctifer, Spotted Guitarfish Assessment by: Ebert, D.A., Khan, M., Ali, M., Akhilesh, K.V. & Jabado, R. View on www.iucnredlist.org Citation: Ebert, D.A., Khan, M., Ali, M., Akhilesh, K.V. & Jabado, R. 2017. Rhinobatos punctifer. The IUCN Red List of Threatened Species 2017: e.T161447A109904426. http://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T161447A109904426.en Copyright: © 2017 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Chondrichthyes Rhinopristiformes Rhinobatidae Taxon Name: Rhinobatos punctifer Compagno & Randall, 1987 Common Name(s): • English: Spotted Guitarfish Taxonomic Source(s): Compagno, L. -

Pontoporia Blainvillei
1 Taller Regional de Evaluación del Estado de Conservación de Especies para el Mar Patagónico según criterios de la Lista Roja de UICN: CONDRICTIOS. Buenos Aires, ARGENTINA - 2017 Results of the 2017 IUCN Regional Red List Workshop for Species of the Patagonian Sea: CHONDRICHTHYANS. Septiembre 2020 Con el apoyo de: 2 PARTICIPANTES DEL TALLER: Daniel Figueroa Universidad Nacional de Mar del Plata, Argentina. Departamento de Biología Marina y Millennium Nucleus for Ecology and Enzo Acuña Sustainable Management of Oceanic Islands (ESMOI), Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile. División Ictiología, Museo Argentino Ciencias Naturales Bernardino Gustavo Chiaramonte Rivadavia (MACN), Argentina. Wildlife Conservation Society, Programa Marino, Argentina. Universidad Juan Martín Cuevas Nacional de La Plata (UNLP), Argentina. Laura Paesch Dirección Nacional de Recursos Acuáticos DINARA, Uruguay Estación Hidrobiológica de Puerto Quequén. Museo Argentino Ciencias Marilú Estalles Naturales Bernardino Rivadavia (MACN), Argentina. Centro de Investigación Aplicada y Transferencia Tecnológica en Marina Coller Recursos Marinos Almirante Storni (CIMAS), Argentina. Mirta García Universidad Nacional de La Plata (UNLP), Argentina. Secretaría de Pesca, Provincia de Chubut. Instituto de Hidrobiología de Nelson Bovcon la UNPSB (Chubut), Argentina. CEPSUL, Instituto Chico Mendes de Conservação da Biodiversidade, Roberta Santos Aguiar Brasília, Brasil. Santiago Montealegre Quijano Universidade Estadual Paulista "Julio de Mesquita Filho" -

Sharks, Rays and Abortion the Prevalence of Capture-Induced
Biological Conservation 217 (2018) 11–27 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Review Sharks, rays and abortion: The prevalence of capture-induced parturition in T elasmobranchs ⁎ Kye R. Adamsa, , Lachlan C. Fetterplacea,c, Andrew R. Davisa, Matthew D. Taylorb, Nathan A. Knottb a School of Biological Sciences, University of Wollongong, Northfields Avenue, Wollongong, NSW 2522, Australia b New South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Locked Bag 1, Nelson Bay, NSW 2315, Australia c Fish Thinkers Research, 11 Riverleigh Avenue, Gerroa, NSW, 2534, Australia ABSTRACT The direct impacts of fishing on chondrichthyans (sharks, rays and chimeras) are well established. Here we review a largely unreported, often misinterpreted and poorly understood indirect impact of fishing on these animals — capture-induced parturition (either premature birth or abortion). Although direct mortality of dis- carded sharks and rays has been estimated, the prevalence of abortion/premature birth and subsequent gen- erational mortality remains largely unstudied. We synthesize a diffuse body of literature to reveal that a con- servative estimate of > 12% of live bearing elasmobranchs (n = 88 species) show capture-induced parturition. For those species with adequate data, we estimate capture-induced parturition events ranging from 2 to 85% of pregnant females (average 24%). To date, capture-induced parturition has only been observed in live-bearing species. We compile data on threat-levels, method of capture, reproductive mode and gestation extent of pre- mature/aborted embryos. We also utilise social media to identify 41 social-media links depicting a capture- induced parturition event which provide supplementary visual evidence for the phenomenon. -

Movements Patterns and Population Dynamics Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) M O V E M E NT PA T T E RNS A ND POPU L A T I O N D Y N A M I CS O F F O UR C A TSH AR KS E ND E M I C T O SO U T H A F RI C A Submitted in fulfilment of the requirements for the degree of M AST E R O F SC I E N C E at R H O D ES UNI V E RSI T Y by JESSI C A ESC O B A R-PO RR AS December 2009 Escobar-Porras, J. 2009 A BST R A C T Sharks are particularly vulnerable to over-exploitation. Although catsharks are an important component of the near-shore marine biodiversity in South Africa and most of the species are endemic, little is known about their movement patterns, home range and population size. With an increasing number of recreational fishers this information is crucial for their conservation. The aims of this study were threefold. Firstly, to identify and analyze existing data sources on movement patterns and population dynamics for four catshark species: pyjama (Poroderma africanum), leopard (P. pantherinum), puffadder (Haploblepharus edwarsii) and brown (H. fuscus). This highlighted a number of shortcomings with existing data sets, largely because these studies had diverse objectives and were not aimed solely at catsharks. Secondly, a dedicated study was carried out for a limited area, testing a number of methods for data collection, and where appropriate the data was analyzed to determine movement patterns and population numbers. -

Appendix 1. South African Marine Bioregions K. Sink, J. Harris and A. Lombard 1. Introduction Biogeography Is Defined As The
Appendix 1. South African marine bioregions K. Sink, J. Harris and A. Lombard 1. Introduction Biogeography is defined as the study of biological life in a spatial and temporal context and is concerned with the analysis and explanation of patterns of distribution (Cox and Moore 1998). An important application of biogeographic studies is the generation of knowledge necessary to achieve adequate and representative conservation of all elements of biodiversity. Conservation of biodiversity pattern requires that a viable proportion of any habitat or species in each biogeographically distinct area is protected, either within a protected area or by management measures that mitigate threats. It is therefore important to realise that any habitat or species in each biogeographic region is seen as distinct and deserving of protection. It is thus recommended that representative marine protected areas need to be established within each principal biogeographic region in South Africa (Hockey and Buxton 1989; Hockey and Branch 1994, 1997; Turpie et al. 2000; Roberts et al. 2003a; Roberts et al. 2003b). A prerequisite to achieving this goal is, however, a knowledge of where biogeographic regions begin and end. For this project, distinct biogeographic areas with clear boundaries were needed for the South African marine environment. The area of interest was from Lüderitz in Namibia to Inhaca Island in Mozambique. 2. Marine biogeographic patterns in South Africa Many studies have investigated marine biogeographic patterns around the coast of South Africa (e.g. Stephenson and Stephenson 1972; Brown and Jarman 1978; Emanuel et al. 1992; Engledow et al. 1992; Stegenga and Bolton 1992; Bustamante and Branch 1996; Bolton and Anderson 1997; Turpie et al. -

Thesis Sci 2021 De Vos Lauren.Pdf
BIODIVERSITY PATTERNS IN FALSE BAY: AN ASSESSMENT USING UNDERWATER CAMERAS Lauren De Vos Thesis Presented for the Degree of DOCTOR OF PHILOSOPHY Universityin the Department ofof Biological Cape Sciences Town UNIVERSITY OF CAPE TOWN October 2020 Supervised by Associate Professor Colin Attwood, Dr Anthony Bernard and Dr Albrecht Götz The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town ii DECLARATIONS PLAGIARISM DECLARATION I know the meaning of plagiarism and declare that all the work in this thesis, save for that which is properly acknowledged, is my own. This thesis has not been submitted in whole or in part for a degree at any other university. Signature: Date: 31 October 2020 iii RESEARCH DECLARATION Research was conducted inside the Table Mountain National Park (TMNP) marine protected area (MPA) with permission from South African National Parks (SANParks). Permit Number: CRC-2014-012. Portions of the fish baited remote underwater mono-video system (mono-BRUVs) data, those pertaining to chondrichthyans, used in this thesis are published in De Vos, L., Watson, R.G.A., Götz, A. & Attwood, C.G. 2015. Baited remote underwater video system (BRUVs) survey of chondrichthyan diversity in False Bay, South Africa. African Journal of Marine Science. 37(2): 209-218. -

Iotc–2014–Wpeb10–Inf20 Sri Lanka National Plan Of
IOTC–2014–WPEB10–INF20 SRI LANKA NATIONAL PLAN OF ACTION FOR THE CONSERVATION AND MANAGEMENT OF SHARKS (SL-NPOA-SHARKS) MINISTRY OF FISHERIES AND AQUATIC RESOURCES DEVELOPMENT DEPARTMENT OF FISHERIES AND AQUATIC RESOURCES NATIONAL AQUATIC RESOURCES RESEARCH AND DEVELOPMENT AGENCY SRI LANKA December, 2013 1 IOTC–2014–WPEB10–INF20 CONTENTS ABBREVIATIONS ………………………………………………………………………………………………………………3 EXECUTIVE SUMMARY……………………………………………………………………………………………………..4 1. INTRODUCTION…………………………………………………………………………………………………………….6 1.1. International Initiatives for the Conservation and Management of Sharks...6 1.2. Development of the Sri Lanka National Plan of Action for the Conservation and Management of Sharks (SLNPOA-Sharks)………………………………………………………………8 2. THE SHARK FISHERY IN SRI LANKA…………………………………………………………………………………8 2.1. The Pelagic Shark Fishery………………………………………………………………………………..9 2.1.1. Pelagic Shark Landings……………………………………………………………………..10 2.1.2. Pelagic Shark Landings by Species…………………………………………………….12 2.1.3 Pelagic Shark Landings by Geographical Area……………………………………13 2.2. The Coastal Thresher Shark Fishery………………………………………………………………..15 2.3. The Spiny Dogfish Shark Fishery……………………………………………………………………..15 2.4. The Skate and Ray Fishery………………………………………………………………………………15 2.5. Utilization, Market and Trade…………………………………………………………………………15 2.6. Discards………………………………………………………………………………………………………….16 2.7. Non - Consumptive Use of Sharks (Eco-tourism)……………………………………………16 3. LEGAL AND ADMINISTRATIVE FRAMEWORK FOR THE CONSERVATION AND MANAGEMENT OF SHARKS……………………………………………………………………………………………………………………….16 -
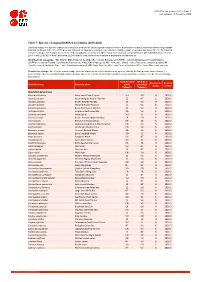
Table 7: Species Changing IUCN Red List Status (2019-2020)
IUCN Red List version 2020-3: Table 7 Last Updated: 10 December 2020 Table 7: Species changing IUCN Red List Status (2019-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category -

Table 7: Species Changing IUCN Red List Status (2019-2020)
IUCN Red List version 2020-2: Table 7 Last Updated: 10 July 2020 Table 7: Species changing IUCN Red List Status (2019-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category