Wood Decay by Inonotus Rickii and Bjerkandera Adusta: a Micro- and Ultra-Structural Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: a Review
Review The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review Andreia Silva, Cristina Delerue-Matos, Sónia A. Figueiredo and Olga M. Freitas * REQUIMTE/LAQV, Instituto Superior de Engenharia do Porto, Rua Dr. António Bernardino de Almeida 431, 4200-072 Porto, Portugal * Correspondence: [email protected] Received: 3 July 2019; Accepted: 25 July 2019; Published: 27 July 2019 Abstract: The occurrence and fate of pharmaceuticals in the aquatic environment is recognized as one of the emerging issues in environmental chemistry. Conventional wastewater treatment plants (WWTPs) are not designed to remove pharmaceuticals (and their metabolites) from domestic wastewaters. The treatability of pharmaceutical compounds in WWTPs varies considerably depending on the type of compound since their biodegradability can differ significantly. As a consequence, they may reach the aquatic environment, directly or by leaching of the sludge produced by these facilities. Currently, the technologies under research for the removal of pharmaceuticals, namely membrane technologies and advanced oxidation processes, have high operation costs related to energy and chemical consumption. When chemical reactions are involved, other aspects to consider include the formation of harmful reaction by-products and the management of the toxic sludge produced. Research is needed in order to develop economic and sustainable treatment processes, such as bioremediation and biosorption. The use of low-cost materials, such as biological matrices (e.g., algae and fungi), has advantages such as low capital investment, easy operation, low operation costs, and the non-formation of degradation by-products. An extensive review of existing research on this subject is presented. -

Oxalic Acid Degradation by a Novel Fungal Oxalate Oxidase from Abortiporus Biennis Marcin Grąz1*, Kamila Rachwał2, Radosław Zan2 and Anna Jarosz-Wilkołazka1
Vol. 63, No 3/2016 595–600 http://dx.doi.org/10.18388/abp.2016_1282 Regular paper Oxalic acid degradation by a novel fungal oxalate oxidase from Abortiporus biennis Marcin Grąz1*, Kamila Rachwał2, Radosław Zan2 and Anna Jarosz-Wilkołazka1 1Department of Biochemistry, Maria Curie-Skłodowska University, Lublin, Poland; 2Department of Genetics and Microbiology, Maria Curie-Skłodowska University, Lublin, Poland Oxalate oxidase was identified in mycelial extracts of a to formic acid and carbon dioxide (Mäkelä et al., 2002). basidiomycete Abortiporus biennis strain. Intracellular The degradation of oxalate via action of oxalate oxidase enzyme activity was detected only after prior lowering (EC 1.2.3.4), described in our study, is atypical for fun- of the pH value of the fungal cultures by using oxalic or gi and was found predominantly in higher plants. The hydrochloric acids. This enzyme was purified using size best characterised oxalate oxidase originates from cereal exclusion chromatography (Sephadex G-25) and ion-ex- plants (Dunwell, 2000). Currently, only three oxalate oxi- change chromatography (DEAE-Sepharose). This enzyme dases of basidiomycete fungi have been described - an exhibited optimum activity at pH 2 when incubated at enzyme from Tilletia contraversa (Vaisey et al., 1961), the 40°C, and the optimum temperature was established at best characterised so far enzyme from Ceriporiopsis subver- 60°C. Among the tested organic acids, this enzyme ex- mispora (Aguilar et al., 1999), and an enzyme produced by hibited specificity only towards oxalic acid. Molecular Abortiporus biennis (Grąz et al., 2009). The enzyme from mass was calculated as 58 kDa. The values of Km for oxa- C. -

Identification and Tracking Activity of Fungus from the Antarctic Pole on Antagonistic of Aquatic Pathogenic Bacteria
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 19F–079/2019/22–6–1311–1319 DOI: 10.17957/IJAB/15.1203 http://www.fspublishers.org Full Length Article Identification and Tracking Activity of Fungus from the Antarctic Pole on Antagonistic of Aquatic Pathogenic Bacteria Chuner Cai1,2,3, Haobing Yu1, Huibin Zhao2, Xiaoyu Liu1*, Binghua Jiao1 and Bo Chen4 1Department of Biochemistry and Molecular Biology, College of Basic Medicine, Naval Medical University, Shanghai, 200433, China 2College of Marine Ecology and Environment, Shanghai Ocean University, Shanghai, 201306, China 3Co-Innovation Center of Jiangsu Marine Bio-industry Technology, Lianyungang, Jiangsu, 222005, China 4Polar Research Institute of China, Shanghai, 200136, China *For correspondence: [email protected] Abstract To seek the lead compound with the activity of antagonistic aquatic pathogenic bacteria in Antarctica fungi, the work identified species of a previously collected fungus with high sensitivity to Aeromonas hydrophila ATCC7966 and Streptococcus agalactiae. Potential active compounds were separated from fermentation broth by activity tracking and identified in structure by spectrum. The results showed that this fungus had common characteristics as Basidiomycota in morphology. According to 18S rDNA and internal transcribed space (ITS) DNA sequencing, this fungus was identified as Bjerkandera adusta in family Meruliaceae. Two active compounds viz., veratric acid and erythro-1-(3, 5-dichlone-4- methoxyphenyl)-1, 2-propylene glycol were identified by nuclear magnetic resonance spectrum and mass spectrum. Veratric acid was separated for the first time from any fungus, while erythro-1-(3, 5-dichlone-4-methoxyphenyl)-1, 2-propylene glycol was once reported in Bjerkandera. -

Author Template for Journal Articles
Metal phytoremediation and nutrient accumulation Kaur et al. Int J Env Tech Sci 7: 249–252 © 2019 http://www.kalyanishineindia.org/index.php/journal ORIGINAL ARTICLE Kalyani Shine India Bioaccumulation of several trace metal elements in fungi collected from Gharbia Governorate, Egypt Mohamed Y A Bedaiwy, Yehia A-G Mahmoud Tanta University, Faculty of Science, Botany Department, Mycology Research Laboratory, Tanta 31527, Egypt ARTICLE INFO ABSTRACT Article history: The determination of trace metal elements in fungi is important from an economic Received– 01 March, 2019 point of view, since fungi are used for human or animal consumption and/or as Revised– 01 April, 2019 biofertilizers for plants. Furthermore, trace metal concentrations could be a good Accepted– 02 May, 2019 indicator for soil heath and contamination due to human interference. The present Available– 12 May, 2019 study determined the silver, cadmium, cobalt, chromium, nickel, lead, selenium, (online) zinc and aluminium concentrations in the sporocarps of wild Ganoderma Keywords: resinaceum, Bjerkandera adusta, Ganoderma applanatum, Inonotus radiatus and Metals Abortiporus biennis collected from Gharbia Governorate, Egypt, during 2017- Contamination 2018. The highest zinc concentrations, i.e., values of 39.10 and 42.35 mg kg-1, Sporocarp were recorded in fungi sporocarps gathered from the Zefta and Alsantah urban Ganoderma sp. areas of Gharbia Governorate, respectively. Neither cadmium nor cobalt was Bjerkandera sp. observed in the sporocarps of the five species gathered during this investigation Ganoderma sp. period. The aluminium contents of the five studied higher fungi species were Inonotus sp. higher than the other trace metals contents of these fungi, reaching 3058 mg kg-1 Abortiporus sp. -

Aegis Boa (Polyporales, Basidiomycota) a New Neotropical Genus and Species Based on Morphological Data and Phylogenetic Evidences
Mycosphere 8(6): 1261–1269 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/6/11 Copyright © Guizhou Academy of Agricultural Sciences Aegis boa (Polyporales, Basidiomycota) a new neotropical genus and species based on morphological data and phylogenetic evidences Gómez-Montoya N1, Rajchenberg M2 and Robledo GL 1, 3* 1 Laboratorio de Micología, Instituto Multidisciplinario de Biología Vegetal, Consejo Nacional de Investigaciones Científicas y Técnicas, Universidad Nacional de Córdoba, CC 495, CP 5000 Córdoba, Argentina 2 Centro de Investigación y Extensión Forestal Andino Patagónico (CIEFAP), C.C. 14, 9200 Esquel, Chubut and Universidad Nacional de la Patagonia S.J. Bosco, Ingeniería Forestal, Ruta 259 km 14.6, Esquel, Chubut, Argentina 3 Fundación FungiCosmos, Av. General Paz 54, 4to piso, of. 4, CP 5000, Córdoba, Argentina. Gómez-Montoya N, Rajchenberg M, Robledo GL. 2017 – Aegis boa (Polyporales, Basidiomycota) a new neotropical genus and species based on morphological data and phylogenetic evidences. Mycosphere 8(6), 1261–1269, Doi 10.5943/mycosphere/8/6/11 Abstract The new genus, Aegis Gómez-Montoya, Rajchenb. & Robledo, is described to accommodate the new species Aegis boa based on morphological data and phylogenetic evidences (ITS – LSU rDNA). It is characterized by a particular monomitic hyphal system with thick-walled, widening, inflated and constricted generative hyphae, and allantoid basidiospores. Phylogenetically Aegis is closely related to Antrodiella aurantilaeta, both species presenting an isolated position within Polyporales into Grifola clade. The new taxon is so far known from Yungas Mountain Rainforests of NW Argentina. Key words – Grifola – Neotropical polypores – Tyromyces Introduction Polypore diversity of NW Argentina was reviewed by Robledo & Rajchenberg (2007). -

Index to Volumes 1-20 (1950-1980)
Karstenia 20: 33- 79. 1981 Index to volumes 1-20 (1950-1980) Compiled by HILKKA KOPONEN, LIISA SEPPANEN and PENTTI ALANKO Editor's note nish Mycological Society, Sienilehti (earlier Sienitie toja-Svampnytt). Unfortunately the exact dates of issue could not be This first cumulative Index of Karstenia covers all the traced for the early numbers of Karstenia. For the papers so far published in the magazine. Karstenia, first eight volumes only the year of printing is known, dedicated to the famous Finnish mycologist Petter for volumes 9-14 we know the month and year of Adolf Karsten (1834-1917), was first published at ir printing. From vol. 15 onwards the exact date of pu regular intervals, between the years 1950-1976, and blication has been recorded. These dates are found on each issue was numbered as a separate volume (l-16). the covers of the issues, and are not repeated here. From volume 17 onwards, Karstenia has come out on It is hoped that this Index will prove a great help to an annual basis, each volume consisting of two issues those using the issues of Karstenia. As the Editor of (plus a supplement to vol. 18). Karstenia, I wish to express my best thanks to Mrs. During this time Karstenia has established its posi Hilkka Koponen, Lic.Phil., Miss Liisa Seppanen, tion as an important medium for the studies of Fin M.Sc., and Mr. Pentti Alanko, Head Gardener (all nish mycologists. Writers from abroad have contribu from the Department of Botany, University of Hel ted only occasionally, and mainly on topics in some sinki), who have painstakingly performed the labori way connected with Finland. -

A Revised Family-Level Classification of the Polyporales (Basidiomycota)
fungal biology 121 (2017) 798e824 journal homepage: www.elsevier.com/locate/funbio A revised family-level classification of the Polyporales (Basidiomycota) Alfredo JUSTOa,*, Otto MIETTINENb, Dimitrios FLOUDASc, € Beatriz ORTIZ-SANTANAd, Elisabet SJOKVISTe, Daniel LINDNERd, d €b f Karen NAKASONE , Tuomo NIEMELA , Karl-Henrik LARSSON , Leif RYVARDENg, David S. HIBBETTa aDepartment of Biology, Clark University, 950 Main St, Worcester, 01610, MA, USA bBotanical Museum, University of Helsinki, PO Box 7, 00014, Helsinki, Finland cDepartment of Biology, Microbial Ecology Group, Lund University, Ecology Building, SE-223 62, Lund, Sweden dCenter for Forest Mycology Research, US Forest Service, Northern Research Station, One Gifford Pinchot Drive, Madison, 53726, WI, USA eScotland’s Rural College, Edinburgh Campus, King’s Buildings, West Mains Road, Edinburgh, EH9 3JG, UK fNatural History Museum, University of Oslo, PO Box 1172, Blindern, NO 0318, Oslo, Norway gInstitute of Biological Sciences, University of Oslo, PO Box 1066, Blindern, N-0316, Oslo, Norway article info abstract Article history: Polyporales is strongly supported as a clade of Agaricomycetes, but the lack of a consensus Received 21 April 2017 higher-level classification within the group is a barrier to further taxonomic revision. We Accepted 30 May 2017 amplified nrLSU, nrITS, and rpb1 genes across the Polyporales, with a special focus on the Available online 16 June 2017 latter. We combined the new sequences with molecular data generated during the Poly- Corresponding Editor: PEET project and performed Maximum Likelihood and Bayesian phylogenetic analyses. Ursula Peintner Analyses of our final 3-gene dataset (292 Polyporales taxa) provide a phylogenetic overview of the order that we translate here into a formal family-level classification. -

Polyporaceae of Iowa: a Taxonomic, Numerical and Electrophoretic Study Robert John Pinette Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1983 Polyporaceae of Iowa: a taxonomic, numerical and electrophoretic study Robert John Pinette Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Pinette, Robert John, "Polyporaceae of Iowa: a taxonomic, numerical and electrophoretic study " (1983). Retrospective Theses and Dissertations. 8954. https://lib.dr.iastate.edu/rtd/8954 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Fungal Biodiversity in Extreme Environments and Wood Degradation Potential
http://waikato.researchgateway.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. Fungal biodiversity in extreme environments and wood degradation potential A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biological Sciences at The University of Waikato by Joel Allan Jurgens 2010 Abstract This doctoral thesis reports results from a multidisciplinary investigation of fungi from extreme locations, focusing on one of the driest and thermally broad regions of the world, the Taklimakan Desert, with comparisons to polar region deserts. Additionally, the capability of select fungal isolates to decay lignocellulosic substrates and produce degradative related enzymes at various temperatures was demonstrated. The Taklimakan Desert is located in the western portion of the People’s Republic of China, a region of extremes dominated by both limited precipitation, less than 25 mm of rain annually and tremendous temperature variation. -

Polypore Communities in Broadleaved Boreal Forests
Silva Fennica 46(3) research articles SILVA FENNICA www.metla.fi/silvafennica · ISSN 0037-5330 The Finnish Society of Forest Science · The Finnish Forest Research Institute Polypore Communities in Broadleaved Boreal Forests Anni Markkanen and Panu Halme Markkanen, A. & Halme, P. 2012. Polypore communities in broadleaved boreal forests. Silva Fennica 46(3): 317–331. The cover and extent of boreal broadleaved forests have been decreasing due to modern forest management practices and fire suppression. As decomposers of woody material, polypores are ecologically important ecosystem engineers. The ecology and conservation biology of polypores have been studied intensively in boreal coniferous forests. However, only a few studies have focused on the species living on broadleaved trees. To increase knowledge on this species group we conducted polypore surveys in 27 broadleaved forests and 303 forest compartments (539 ha) on the southern boreal zone in Finland and measured dead wood and forest characteristics. We detected altogether 98 polypore species, of which 13 are red-listed in Finland. 60% of the recorded species are primarily associated with broadleaved trees. The number of species in a local community present in a broadleaved forest covered approximately 50 species, of which 30–40 were primarily associated with broadleaved trees. The size of the inventoried area explained 67% of the variation in the species richness, but unlike in previ- ous studies conducted in coniferous forests, dead wood variables as well as forest structure had very limited power in explaining polypore species richness on forest stand level. The compartments occupied by red listed Protomerulius caryae had an especially high volume of living birch, but otherwise the occurrences of red-listed species could not be predicted based on the forest structure. -

Polyporales, Basidiomycota)
https://helda.helsinki.fi Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota) Miettinen, Otto 2016 Miettinen , O , Spirin , V , Vlasák , J , Rivoire , B , Stenroos , S & Hibbett , D 2016 , ' Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota) ' MycoKeys , vol. 17 , pp. 1-46 . DOI: 10.3897/mycokeys.17.10153 http://hdl.handle.net/10138/170328 https://doi.org/10.3897/mycokeys.17.10153 Downloaded from Helda, University of Helsinki institutional repository. This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Please cite the original version. A peer-reviewed open-access journal MycoKeys 17:Polypores 1–46 (2016) and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota) 1 doi: 10.3897/mycokeys.17.10153 RESEARCH ARTICLE MycoKeys http://mycokeys.pensoft.net Launched to accelerate biodiversity research Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota) Otto Miettinen1, Viacheslav Spirin1, Josef Vlasák2, Bernard Rivoire3, Soili Stenroos1, David S. Hibbett4 1 Finnish Museum of Natural History, University of Helsinki, Finland 2 Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic 3 Société Linnéenne, Lyon, France 4 Biology Department, Clark University, Worcester, Massachusetts, United States of America Corresponding author: Otto Miettinen ([email protected]) Academic editor: R.H. Nilsson | Received 19 August 2016 | Accepted 8 November 2016 | Published 8 December 2016 Citation: Miettinen O, Spirin V, Vlasák J, Rivoire B, Stenroos S, Hibbett DS (2016) Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 17: 1–46. https://doi.org/10.3897/mycokeys.17.10153 Abstract We explored whether DNA-phylogeny-based and morphology-based genus concepts can be reconciled in the basidiomycete family Phanerochaetaceae. -
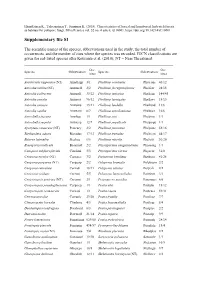
Supplementary File S1
Hämäläinen K., Tahvanainen T., Junninen K. (2018). Characteristics of boreal and hemiboreal herb-rich forests as habitats for polypore fungi. Silva Fennica vol. 52 no. 4 article id 10001. https://doi.org/10.14214/sf.10001 Supplementary file S1 The scientific names of the species, abbreviations used in the study, the total number of occurrences, and the number of sites where the species was recorded. IUCN classifications are given for red-listed species after Kotiranta et al. (2010); NT = Near Threatened. Occ. Occ. Species Abbreviation Species Abbreviation /sites /sites Amylocystis lapponica (NT) Amyllapp 3/1 Phellinus conchatus Phelconc 46/12 Antrodia mellita (NT) Antrmell 2/2 Phellinus ferrugineofuscus Phelferr 28/15 Antrodia pallescens Antrpall 39/22 Phellinus igniarius Pheligni 144/45 Antrodia serialis Antrseri 96/32 Phellinus laevigatus Phellaev 18/15 Antrodia sinuosa Antrsinu 15/11 Phellinus lundellii Phellund 13/6 Antrodia xantha Antrxant 8/7 Phellinus nigrolimitatus Phelnigr 16/6 Antrodiella faginea Antrfagi 1/1 Phellinus pini Phelpini 1/1 Antrodiella serpula Antrserp 12/7 Phellinus populicola Phelpopu 1/1 Aporpium canescens (NT) Protcary 2/2 Phellinus punctatus Phelpunc 58/16 Bjerkandera adusta Bjeradus 17/12 Phellinus tremulae Pheltrem 44/17 Butyrea luteoalba Steclute 6/6 Phellinus viticola Phelviti 36/20 Byssoporia mollicula Byssmoll 2/2 Physisporinus sanguinolentus Physsang 1/1 Canopora subfuscoflavida Cinelind 3/3 Physisporinus vitreus Physvitr 31/8 Ceriporia excelsa (NT) Ceriexce 3/2 Piptoporus betulinus Piptbetu 41/28 Ceriporia