In Rajanganaya Reservoir, Sri Lanka
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hotspots of Land Use/Land Cover Change Around Bolgoda Wetland, Sri Lanka
J.Natn.Sci.Foundation Sri Lanka 2020 48 (3): 219 - 226 DOI: DOI: http://dx.doi.org/10.4038/jnsfsr.v48i3.9376 RESEARCH ARTICLE Hotspots of land use/land cover change around Bolgoda wetland, Sri Lanka AHLCM Athapaththu *, Deepthi Wickramasinghe and MGMC Somachandra Department of Zoology and Environmental Sciences, Faculty of Science, University of Colombo, Colombo. Submitted: 15 August 2019; Revised: 21 January 2020; Accepted: 23 March 2020 Abstract: Wetlands are among the most productive ecosystems habitats are particularly important for livelihood of the in the world. Urban wetlands are increasingly aff ected by communities who live in the area. For instance, fi sheries population growth and developmental activities. A buff er and tourism are dependent on wetlands (Gachhadar region of 60 m from the Bolgoda lake boundary was gazetted et al ., 2004). Ecosystem services provided by wetlands as an Environmental Protection Area (EPA) by the Central include absorption of pollutants, carbon sequestration, Environmental Authority in 2009 as it is the largest freshwater wetland in Sri Lanka. This study attempts to quantify the land groundwater recharge, and disaster mitigation (Chmura use changes during the period of 2001 to 2019 using GIS and et al ., 2003; Chen et al ., 2008; Ramsar Convention to identify the ‘hot spots’ where a signifi cant change in land use Secretariat, 2014). Yet human induced land use/land occurred. Land use/land cover (LULC) changed in the Bolgoda cover (LULC) changes aff ect the wetlands worldwide wetland area disproportionately. The greatest proportion of as well as services provided by them (Zhao et al ., 2004; loss of natural area was observed in dense vegetation, where a Zorrilla-Miras et al ., 2014). -

The Effect of Water Bodies As a Determinant Force in Generating Urban Form
The Effect of Water Bodies as a Determinant Force in Generating Urban Form. A Study on Creating a Symbiosis between the two with a case study of the Beira Lake, City of Colombo. K. Pradeep S. S. Fernando. 139404R Degree of Masters in Urban Design 2016 Department of Architecture University of Moratuwa Sri Lanka The Effect of Water Bodies as a Determinant Force in Generating Urban Form. A Study on Creating a Symbiosis between the two with a case study of the Beira Lake, City of Colombo. K. Pradeep S. S. Fernando. 139404R Degree of Masters in Urban Design 2016 Department of Architecture University of Moratuwa Sri Lanka THE EFFECT OF WATER BODIES AS A DETERMINANT FORCE IN GENERATING URBAN FORM - WITH A STUDY ON CREATING A SYMBIOSIS BETWEEN THE TWO WITH A CASE STUDY OF THE BEIRA LAKE, CITY OF COLOMBO. Water bodies present in Urban Contexts has been a primary determinant force in the urban formation and settlement patterns. With the evolutionary patterns governing the cities, the presence of water bodies has been a primary generator bias, thus being a primary contributor to the character of the city and the urban morphology. Urban form can be perceived as the pattern in which the city is formed where the street patterns and nodes are created, and the 03 dimensional built forms, which holistically forms the urban landscape. The perception of urban form has also been a key factor in the human response to the built massing, and fabric whereby the activity pattern is derived, with the sociological implications. DECLARATION I declare that this my own work and this dissertation does not incorporate without acknowledgment any material previously submitted for a Degree or Diploma in any University or any Institute of Higher Learning and to the best of my knowledge and belief it does not contain any materials previously published or written by another person except where acknowledgement is made in the text. -
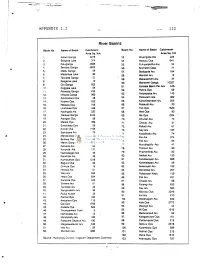
River Basins
APPENDIX I.I 122 River Basins Basin No Name of Basin Catchment Basin No. Name of Basin Catchment Area Sq. Km. Area Sq. Km 1. Kelani Ganga 2278 53. Miyangolla Ela 225 2. Bolgoda Lake 374 54. Maduru Oya 1541 3. Kaluganga 2688 55. Pulliyanpotha Aru 52 4. Bemota Ganga 6622 56. Kirimechi Odai 77 5. Madu Ganga 59 57. Bodigoda Aru 164 6. Madampe Lake 90 58. Mandan Aru 13 7. Telwatte Ganga 51 59. Makarachchi Aru 37 8. Ratgama Lake 10 60. Mahaweli Ganga 10327 9. Gin Ganga 922 61. Kantalai Basin Per Ara 445- 10. Koggala Lake 64 62. Panna Oya 69 11. Polwatta Ganga 233 12. Nilwala Ganga 960 63. Palampotta Aru 143 13. Sinimodara Oya 38 64. Pankulam Ara 382 14. Kirama Oya 223 65. Kanchikamban Aru 205 15. Rekawa Oya 755 66. Palakutti A/u 20 16. Uruhokke Oya 348 67. Yan Oya 1520 17. Kachigala Ara 220 68. Mee Oya 90 18. Walawe Ganga 2442 69. Ma Oya 1024 19. Karagan Oya 58 70. Churian A/u 74 20. Malala Oya 399 71. Chavar Aru 31 21. Embilikala Oya 59 72. Palladi Aru 61 22. Kirindi Oya 1165 73. Nay Ara 187 23. Bambawe Ara 79 74. Kodalikallu Aru 74 24. Mahasilawa Oya 13 75. Per Ara 374 25. Butawa Oya 38 76. Pali Aru 84 26. Menik Ganga 1272 27. Katupila Aru 86 77. Muruthapilly Aru 41 28. Kuranda Ara 131 78. Thoravi! Aru 90 29. Namadagas Ara 46 79. Piramenthal Aru 82 30. Karambe Ara 46 80. Nethali Aru 120 31. -

National Wetland DIRECTORY of Sri Lanka
National Wetland DIRECTORY of Sri Lanka Central Environmental Authority National Wetland Directory of Sri Lanka This publication has been jointly prepared by the Central Environmental Authority (CEA), The World Conservation Union (IUCN) in Sri Lanka and the International Water Management Institute (IWMI). The preparation and printing of this document was carried out with the financial assistance of the Royal Netherlands Embassy in Sri Lanka. i The designation of geographical entities in this book, and the presentation of the material do not imply the expression of any opinion whatsoever on the part of the CEA, IUCN or IWMI concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of the CEA, IUCN or IWMI. This publication has been jointly prepared by the Central Environmental Authority (CEA), The World Conservation Union (IUCN) Sri Lanka and the International Water Management Institute (IWMI). The preparation and publication of this directory was undertaken with financial assistance from the Royal Netherlands Government. Published by: The Central Environmental Authority (CEA), The World Conservation Union (IUCN) and the International Water Management Institute (IWMI), Colombo, Sri Lanka. Copyright: © 2006, The Central Environmental Authority (CEA), International Union for Conservation of Nature and Natural Resources and the International Water Management Institute. Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. -

Y%S ,Xld M%Cd;Dka;%Sl Iudcjd§ Ckrcfha .Eiü M;%H W;S Úfyi the Gazette of the Democratic Socialist Republic of Sri Lanka EXTRAORDINARY
Y%S ,xld m%cd;dka;%sl iudcjd§ ckrcfha .eiÜ m;%h w;s úfYI The Gazette of the Democratic Socialist Republic of Sri Lanka EXTRAORDINARY wxl 2072$58 - 2018 uehs ui 25 jeks isl=rdod - 2018'05'25 No. 2072/58 - FRIDAY, MAY 25, 2018 (Published by Authority) PART I : SECTION (I) — GENERAL Government Notifications SRI LANKA Coastal ZONE AND Coastal RESOURCE MANAGEMENT PLAN - 2018 Prepared under Section 12(1) of the Coast Conservation and Coastal Resource Management Act, No. 57 of 1981 THE Public are hereby informed that the Sri Lanka Coastal Zone and Coastal Resource Management Plan - 2018 was approved by the cabinet of Ministers on 25th April 2018 and the Plan is implemented with effect from the date of Gazette Notification. MAITHRIPALA SIRISENA, Minister of Mahaweli Development and Environment. Ministry of Mahaweli Development and Environment, No. 500, T. B. Jayah Mawatha, Colombo 10, 23rd May, 2018. 1A PG 04054 - 507 (05/2018) This Gazette Extraordinary can be downloaded from www.documents.gov.lk 1A 2A I fldgi ( ^I& fPoh - YS% ,xld m%cd;dka;s%l iudcjd§ ckrcfha w;s úfYI .eiÜ m;%h - 2018'05'25 PART I : SEC. (I) - GAZETTE EXTRAORDINARY OF THE DEMOCRATIC SOCIALIST REPUBLIC OF SRI LANKA - 25.05.2018 CHAPTER 1 1. INTRODUCTION 1.1 THE SCOPE FOR COASTAL ZONE AND COASTAL RESOURCE MANAGEMENT 1.1.1. Context and Setting With the increase of population and accelerated economic activities in the coastal region, the requirement of integrated management focused on conserving, developing and sustainable utilization of Sri Lanka’s dynamic and resources rich coastal region has long been recognized. -

Part I : Introduction
PART I : INTRODUCTION Final Report, Volume II Introduction Main Report Chapter 1 CHAPTER 1 INTRODUCTION 1.1 Background of the Study As the urbanized area of the Colombo Metropolitan Region (CMR) expands into the coastal lowland, the difficulty of drainage is causing an increase in the frequency of flood inundation. The inundation results in damage to property and deterioration of infrastructures as well as a worsening hygiene environment for residents. Implementation of remedial measures to reduce inundation is important to secure lands for future development in the CMR (population 5.3 million at 2001). Since the early 1990’s, the Government of Sri Lanka (GOSL) has been implementing the Greater Colombo Flood Control and Environment Improvement Project, being assisted by an Official Development Assistance Loan from the Government of Japan (GOJ). GOSL has also recognized the need for a comprehensive master plan for storm water drainage in the entire CMR and in September 1999 requested GOJ to undertake a study to formulate such a master plan GOJ agreed to conduct the Study on Storm Water Drainage Plan for the Colombo Metropolitan Region in the Democratic Socialist Republic of Sri Lanka (the Study). The Scope of Work was agreed between the Sri Lanka Land Reclamation and Development Corporation (SLLRDC) of the Ministry of Urban Development, Construction & Public Utilities (presently Ministry of Housing & Plantation Infrastructure) and Japan International Cooperation Agency (JICA) on March 7, 2001. 1.2 Objectives of the Study The objectives of the Study are: 1) To formulate a master plan for storm water drainage in the Colombo Metropolitan Region, 2) To conduct feasibility study on a priority project to be identified in the master plan, and 3) To carry out technology transfer to counterpart personnel in the course of the Study. -

A Contingent Valuation Approach for Bolgoda Lake
Jayasekara and Gunawardena/ Vidyodaya Journal of Science Vol. 23 No. 01 (2020) 61-71 Willingness to Pay for Water Quality: A Contingent Valuation Approach for Bolgoda Lake S.A.G.C. Jayasekara1 and U.A.D.P. Gunawardena2* 1Department of Management Audit, Ministry of Finance, The Secretariat, Colombo 01, Sri Lanka 2Department of Forestry and Environmental Science, University of Sri Jayewardenepura, Nugegoda, Sri Lanka Date Received: 30-05-2020 Date Accepted: 05-06-2020 Abstract Bolgoda lake is known as the largest fresh water body in Colombo Urban Area (CUA) and situated in the Southern boundary of CUA. The lake provides a variety of environmental services including recreation, fisheries production and ground water recharge. Environment of the Bolgoda lake is currently threatened by the disposal of waste by industries. However, there will be an increasing demand for the environmental services produced by the Bolgoda lake in the future. Objectives of the present study are to identify the environmental benefits of the lake and estimate economic values of different user groups for the improvement of water quality. A pilot survey was carried out and five major user groups were identified; fishermen, hotel owners, boat owners, recreational users and indirect users. Selected study area for the present study belongs to five GN divisions from Moratuwa and Panadura DS divisions. Contingent valuation method was applied and users’ willingness to pay (WTP) was elicited on a pre-tested hypothetical market focusing on a pollution control programme. Depending on the WTP values, the 5 user groups were regrouped into two categories, WTP values per household per month for the heavy dependency group was LKR 1,550 while for the less dependency group it was LKR 514.30. -

Fish Assemblage Structure of Two Contrasting Stream Catchments Of
The Open Conservation Biology Journal, 2011, 5, 25-44 25 Open Access Fish Assemblage Structure of Two Contrasting Stream Catchments of the Mahaweli River Basin in Sri Lanka: Hallmarks of Human Exploitation and Implications for Conservation Jayakody A. Sumith*,1,3, Kelly R. Munkittrick1 and N. Athukorale2 1Canadian Rivers Institute and Department of Biology, University of New Brunswick, P.O. Box 5050, Saint John, New Brunswick, E2L 4L5, Canada 2Institute of Fundamental Studies, Hanthana Road, Kandy 20000, Sri Lanka 3Permanent Address: Office of the Registrar of Pesticides, Department of Agriculture, 1056, Getambe, P.O. Box 49, Peradeniya 20400, Sri Lanka Abstract: Patterns of fish community composition in the Mahaweli ichthyological region of Sri Lanka were examined in agricultural tributaries of the Uma-oya catchment of the upper Mahaweli River in comparison to more pristine streams in a nature reserve in the Amban-ganga catchment. The Uma-oya catchment shows characteristics commonly observed in extensive agricultural exploitation such as impaired water quality and altered riparian vegetation. The most abundant fish species in the two regions were Garra ceylonensis, Devario malabaricus, and Rasbora daniconius, although their relative abundance differed between sites. G. ceylonensis and Neomacheilus notostigma were the only endemic fish species in common but the latter has been extremely depauperate. Endemism is higher in the reference sites (62.5%) than agricultural sites (ca. 25%); some of the reference streams showed greater diversity with unique fish species and a few species that have not been recorded previously in the catchment. The ichthyofaunal similarity between two catchments was 39% and fish species diversity was negatively correlated with stream gradients (Pearson correlation (-0.630); r2 = 39.6% p = 0.028). -

Final Report Summary
Sri Lanka Ceylon Electricity Board Development Planning on Optimal Power Generation for Peak Demand in Sri Lanka Final Report Summary February 2015 Japan International Cooperation Agency Electric Power Development Co., Ltd. IL JR 15-022 Development Planning on Optimal Power Generation for Peak Demand in Sri Lanka Final Report Summary TABLE OF CONTENTS Chapter 1 Introduction 1.1 Background of the Project ................................................................................................ 1-1 1.2 Purpose and Scope of Study ............................................................................................. 1-2 1.3 Study Plan ......................................................................................................................... 1-3 1.3.1 Study Procedure ................................................................................................... 1-3 1.3.2 Study Schedule and Study Items ......................................................................... 1-4 Chapter 2 Sri Lanka Power Sector Performance and Development Policy 2.1 Power Sector in Sri Lanka ................................................................................................ 2-1 2.1.1 Power Supply Performance ................................................................................. 2-1 2.1.2 Government Power Sector Policy ........................................................................ 2-2 2.1.3 Organizations for Electricity Supply ................................................................... 2-3 -

National Report of Sri Lanka
NATIONAL REPORT OF SRI LANKA on the Formulation of a Transboundary Diagnostic Analysis and Strategic Action Plan for the Bay of Bengal Large Marine Ecosystem Programme Leslie Joseph – National Consultant LIST OF CONTENTS 1.0 INTRODUCTION................................................................................................................................................. 1 1.1 Aim of the national report ...............................................................................................................................1 1.2 Country background.........................................................................................................................................1 1.3 Geographic divisions used in the analysis ....................................................................................................5 1.4 Marine protected areas .....................................................................................................................................7 2.0 STATUS AND DEVELOPMENT POTENTIAL OF THE COASTAL AND MARINE ENVIRONMENT AND ITS LIVING RESOURCES 2.1 Quality of Coastal water..................................................................................................................................8 2.2 River pollution ................................................................................................................................................10 2.3 Availability and quality of fresh water .......................................................................................................11 -

A Preliminary Study on the Variation of Zooplankton Diversity, Abundance and Density in a Selected Branch of Diyawanna Oya Canal System
OUSL Journal (2015) Vol. 9, (pp. 61-81) A Preliminary Study on the Variation of Zooplankton Diversity, Abundance and Density in a Selected Branch of Diyawanna Oya Canal System * L. G. Ruwini Yasadari Perera Department of Zoology, The Open University of Sri Lanka Abstract Zooplankton make the secondary level in aquatic ecosystems and provide a ready source of food for secondary consumers. Hence any fluctuation in their population will directly affect the food webs, and eventually the whole ecological system. This study was carried out along a main branch of Diyawanna Oya, during the period of February to July, 2003. The objectives of the study were to find out the variation in diversity, abundance and density of zooplankton present in a selected branch of Diyawanna Oya and their correlation with some environmental parameters. Zooplankton & water samples were collected monthly from six stations representing both edges and the middle of the canal. During the study, 39 species of rotifers, 9 species of cladocerans, 6 species of copepods, 5 species of ostracods, some larval stages of malacostracans, 2 species of large protozoans and one Tardigrade species were recorded. There was a significant monthly variation in the average density of zooplankton. It was positively correlated with the flow rate (r = 0.81), depth (r = 0.47) and temperature (r = 0.58) of water. And there was a significant variation in density and abundance among six sampling stations. Density values of stations were positively correlated with DO level (r = 0.72) while it was negatively correlated with total alkalinity (r = -0.78), total hardness (r = -0.67) and conductivity (r = -0.6). -

The Beira Lake's Rich History
H O M E Sunday, 13 November 2011 OBSERVER ONLINE Landmarks: News Editorial The Beira Lake's rich history Finance The Beira Lake is not an unfamiliar place to most of us but very few may be aware of its history - from its Features beginning in the 16th century. With the Government's plans to develop this lake into a tourist zone (News item Political appeared on September 11 in the Junior Observer) we decided to enlighten you about this famous lake and also clear out any misconception you may have had about the origin and history of the Lake. Security Sports I thought that Colombo's Beira Lake was a Spectrum natural lake unlike the Kandy lake which is man- Montage made, until I read a reference to this lake five or six years ago, in a translation of a book by a Impact Portuguese priest, Father Fernao de World Queyroz.Father Queyroz says, "when Vijaya Obituaries Bahu laid seige to Colombo in 1522, the Junior Portuguese captain, Lopo de Britto pursued the Magazine attackers, killing and wounding them, till they reached a stream, which was afterwards OTHER dammed into a lake for the better fortification of PUBLICATIONS the city." OTHER LINKS This "stream" was a distributary of the Kelani Ganga, and was known as Kolon Ganga. A distributary is a stream or small river that flows out of the main river and discharges the water into the sea. River deltas are formed by these distributaries. This small river would have branched off the Kelani Ganga at Sedawatta or thereabouts, flowed along the low- lying land past Dematagoda and into the sea at a point north of the present harbour.