Caring for Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Assessment and Management Framework (EAMF)
Environmental Assessment & Management Framework - SCDP 33333333Environmental Assessment and Public Disclosure Authorized Management Framework Strategic Cities Development Project (SCDP) Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Ministry of Megapolis and Western Development January 2016 January, 2016 Page 1 Environmental Assessment & Management Framework - SCDP Table of Contents CHAPTER 1: PROJECT DESCRIPTION ...........................................................................1 1.1 Project concept & objective ....................................................................................... 1 1.2 Project Description ..................................................................................................... 1 1.3 Objective of the Environmental Assessment and Management Framework (EAMF) ........................................................................................................................ 2 CHAPTER 2: POLICY, LEGAL AND ADMINISTRATIVE FRAMEWORK .............4 2.1 Overview of Environmental Legislation ................................................................ 4 2.2 Detail Review of Key Environmental and Urban Services Related Legislation 5 2.3 World Bank Safeguard Policies .............................................................................. 16 2.4 World Heritage Convention ................................................................................... 21 CHAPTER 3: DESCRIPTION OF THE PROJECT AREA ............................................22 -

Evolutionof Coastallaridformsinthe Western Part of Srilanka
HiroshimaHiroshimaGeographicalAssociation Geographical Association Geographical Sciences vol, 43 no.1 pp, 18-36, 1988 Evolution of Coastal Laridforms inthe Western Part of Sri Lanka JINADASA KATUPOTHA* Key Words:evolution of coastal landforms, SriLanka, late Pleistocene, Holocene, landfOmi classMcation Abstract Geomorphic and geologic evidence shows four different stages {Stage I-IV} in the evolution of coastal landforrns on the west coast of Sri Lanka during the Iate Pleistocene and Holocene Epochs. The author assumes that the old ridges in Stage I at Sembulailarna, Kiriyanl(ailiya, Pambala, Wiraliena, Uluambalarna and Kadrana areas have been fonned precedng the Holocene transgression. Low hMs and ridges in the area were coated mainly by wind blown sand, fonowing the lower sea levels during the Late Pleistocene and Earty Holocene Epochs. Radiocarbon datings en the west and seuth coast$ reveal that the sea level remained 1rn or more above the present sea level between 6170± 70 and 535e± 80 yr B. P. During this transgression, the forTner drainage basins were submerged and headland bay beaches were ereated. Many wetlands aiid beach ridges, particularly in Stages ll, III, and IV were gradualy formed owing to rninor oscMations of sea levet after mid-Holocene. Most of these landiorTns haveaclose relationship with main climatic zenes of the country. 1987; Katupotha, 1988) also help to deterrnine their I. Introduction evolution. The island of Sri Lanka has a coastline over Coastal Iandform maps of the study area were 1920 km in length, exhibiting a diversity of coastal cornpiled by means of interpretation of aerial photo- landromis. The coastal lowlands with elevation graphs (1:40,OOO-Survey Department of Sri Lanka, from mean sea level (MSL) to 30m consist of 1956) and field observations. -

A Strategy for Nature Tourism Management
I I I A STRATEGY FOR NATURE TOURISM I MANAGEMENT: I Review of the EnvIronmental and Economic Benefits I of Nature TourIsm and Measures to Increase these Benefits I By I H M 8 C Herath M Sivakumar I P Steele I FINAL REPORT I August 1997 I Prepared for the Ceylon Tourrst Board and Department of Wildlife I USAIDI Natural Resources & Environmental Polley Project International Resources Group (NAREPP/IRG) I A project of the United States Agency for International Development and the I Government of Sri Lanka I I I I I I I DlScriptlOllS about Authors Mr HMC Herath IS a Deputy DIrector workIng for Department of WIldlIfe I ConservatIon, 18, Gregory's Road, Colombo 07, TP No 94-01-695 045 Mr M Sivakurnar IS a Research asSIStant, EnvIronmental DIvISIon Mmistry of I Forestry and EnvIronment, 3 rd Floor, Umty Plaza Bmldmg, Colombo 04 Mr Paul Steele IS an EconomIC Consultant workIng for EnvIronmental DIvISIon, I MllliStry of Forestry and EnvIronment, 3 rd Floor, Umty Plaza BUlldmg, Colombo 04 I I I I I I I I I I I I I I I I CONTENTS I Page I Executive Summary 1-11 1 IntroductIOn 12 I 2 EXIstmg market for nature tounsm 13-19 I 3 Survey of eXIstIng nature tounsm sItes 20-35 4 EnvIronmental and economIC ObjectIves of a I nature tounsm management strategy 36-42 5 QuantIfymg the economiC benefits from nature tounsm 43-56 I 6 ActI\ ltles and SItes for dIversIfymg and expandIng nature tounsm 57-62 I 7 ConclUSIOns and RecommendatIons for IncreasIng the e'1\ Ironmental and economIC benefits of I nature tounsm 63-65 8 References 66 I 9 Annex 1 LIst of persons consulted 67-68 I Annex 2 Graphs of VIsItor entrance and revenues 69-77 Annex 3 Summary of RecommendatIons of Nature Tounsm Workshop and LISt I of PartIcipants 78-80 I I I I I I I Executive summary I 1 Nature tOUrIsm should be promoted by the Ceylon TourlSt Board to mcrease the number of tourlSts vlSlt10g Sn Lanka. -

Project for Formulation of Greater Kandy Urban Plan (Gkup)
Ministry of Megapolis and Western Development Urban Development Authority Government of the Democratic Socialist Republic of Sri Lanka PROJECT FOR FORMULATION OF GREATER KANDY URBAN PLAN (GKUP) Final Report Volume 2: Main Text September 2018 Japan International Cooperation Agency (JICA) Oriental Consultants Global Co., Ltd. NIKKEN SEKKEI Research Institute EI ALMEC Corporation JR 18-095 Ministry of Megapolis and Western Development Urban Development Authority Government of the Democratic Socialist Republic of Sri Lanka PROJECT FOR FORMULATION OF GREATER KANDY URBAN PLAN (GKUP) Final Report Volume 2: Main Text September 2018 Japan International Cooperation Agency (JICA) Oriental Consultants Global Co., Ltd. NIKKEN SEKKEI Research Institute ALMEC Corporation Currency Exchange Rate September 2018 LKR 1 : 0.69 Yen USD 1 : 111.40 Yen USD 1 : 160.83 LKR Map of Greater Kandy Area Map of Centre Area of Kandy City THE PROJECT FOR FORMULATION OF GREATER KANDY URBAN PLAN (GKUP) Final Report Volume 2: Main Text Table of Contents EXECUTIVE SUMMARY PART 1: INTRODUCTION CHAPTER 1 INTRODUCTION ........................................................................... 1-1 1.1 Background .............................................................................................. 1-1 1.2 Objective and Outputs of the Project ....................................................... 1-2 1.3 Project Area ............................................................................................. 1-3 1.4 Implementation Organization Structure ................................................... -

Fisheries Management Provisions
FISHERIES INSTITUTIONAL ANALYSIS AND CAPACITY ASSESSMENT TO THE MINISTRY OF FISHERIES AND AQUATIC RESOURCES, SRI LANKA APPENDIX I: Fisheries Management provisions Table I.1: Fisheries co-management principles Participatory Fisheries Resource Meaning Management Principles The spirit of governance and administration are the interests of the people of Sri Lanka, based on their own aspirations. Department of Fisheries and Aquatic Under decentralization of the fisheries management process, DFAR and the District Fisheries Offices are the responsible Resources is responsible for facilitating the stakeholders: the decision-makers. Hence, these regional fisheries agencies are also responsible for facilitating the management of national and coastal fisheries management of regional fisheries resources by providing human and financial resources to support PFRM as a resources. framework for the management of regional and national fisheries resources. Stakeholders are the participants of fisheries management. The spirit of decentralization of decision-making is that stakeholders should decide on how their aspirations can be met. Stakeholders include: fishermen using different gear types; fish traders; fish processors; fisheries scientists and researchers; coastal communities; fish and plant farmers; district fisheries agencies and the central and district government fisheries agency (DFAR). Stakeholders of participatory coastal fisheries resource management are the coastal The selection of the appropriate stakeholder groups, to be involved in fisheries resource management, should be carried communities, private sectors and government out through stakeholder analysis and the best people to represent these groups chosen democratically. Stakeholder agencies. representatives must have the confidence of the group they represent to ensure ownership of decisions and the empowerment of the stakeholder groups. The social and cultural differences of stakeholders should be formally accepted as input into the decision making process. -

The Government of the Democratic
THE GOVERNMENT OF THE DEMOCRATIC SOCIALIST REPUBLIC OF SRI LANKA FINANCIAL STATEMENTS OF THE GOVERNMENT FOR THE YEAR ENDED 31ST DECEMBER 2019 DEPARTMENT OF STATE ACCOUNTS GENERAL TREASURY COLOMBO-01 TABLE OF CONTENTS Page No. 1. Note to Readers 1 2. Statement of Responsibility 2 3. Statement of Financial Performance for the Year ended 31st December 2019 3 4. Statement of Financial Position as at 31st December 2019 4 5. Statement of Cash Flow for the Year ended 31st December 2019 5 6. Statement of Changes in Net Assets / Equity for the Year ended 31st December 2019 6 7. Current Year Actual vs Budget 7 8. Significant Accounting Policies 8-12 9. Time of Recording and Measurement for Presenting the Financial Statements of Republic 13-14 Notes 10. Note 1-10 - Notes to the Financial Statements 15-19 11. Note 11 - Foreign Borrowings 20-26 12. Note 12 - Foreign Grants 27-28 13. Note 13 - Domestic Non-Bank Borrowings 29 14. Note 14 - Domestic Debt Repayment 29 15. Note 15 - Recoveries from On-Lending 29 16. Note 16 - Statement of Non-Financial Assets 30-37 17. Note 17 - Advances to Public Officers 38 18. Note 18 - Advances to Government Departments 38 19. Note 19 - Membership Fees Paid 38 20. Note 20 - On-Lending 39-40 21. Note 21 (Note 21.1-21.5) - Capital Contribution/Shareholding in the Commercial Public Corporations/State Owned Companies/Plantation Companies/ Development Bank (8568/8548) 41-46 22. Note 22 - Rent and Work Advance Account 47-51 23. Note 23 - Consolidated Fund 52 24. Note 24 - Foreign Loan Revolving Funds 52 25. -

Private Touring & Villas
PRIVATE TOURING & VILLAS Jaffna Palk Strait Open to visitors for the first time in 30 years INDIA Jaffna Delft Island CONTENTS About Us................................................. 4 – 5 Private Touring in Sri Lanka ............... 6 – 7 Sri Lanka Accommodation .............. 8 – 15 Kuchchaveli Mannar A sun drenched beach for A Taste of Sri Lanka ........................ 16 – 17 10 months of the year Gulf Family Holiday Sri Lanka ................ 18 – 19 of Mannar Kuchchaveli Active Sri Lanka ................................ 20 – 21 Anuradhapura Quintessential Sri Lanka ................ 22 – 23 Wilpattu NP Join pilgrims at the sacred Bodhi tree Trincomalee Sri Lanka’s largest Wildlife of Sri Lanka ........................ 24 – 25 national park Sri Lankan Family Adventure ........ 26 – 27 Wilpattu NP Anuradhapura Classic Sri Lanka .............................. 28 – 29 Thirappane Kaudulla Sri Lanka In Style ............................. 30 – 31 Sigiriya Ritigala Climb the Minneriya Celebrate Sri Lanka ......................... 32 – 33 imposing Sigiriya Habarana Rock Fortress Indian Ocean Puttalam I Need A Tropical Holiday .............. 34 – 35 Pidurangala Sigiriya Polonnaruwa Surf Sri Lanka ................................... 36 – 37 Dambulla Honeymoon Sri Lanka .................... 38 – 39 Batticaloa Backroads of Sri Lanka................... 40 – 41 Kandy SRI LANK A Home to the Secret Sri Lanka ............................... 42 – 43 UNESCO Temple of Makandura the Sacred Tooth Gal Oya Discover an idyllic Villas of Sri Lanka ........................... -

Hotspots of Land Use/Land Cover Change Around Bolgoda Wetland, Sri Lanka
J.Natn.Sci.Foundation Sri Lanka 2020 48 (3): 219 - 226 DOI: DOI: http://dx.doi.org/10.4038/jnsfsr.v48i3.9376 RESEARCH ARTICLE Hotspots of land use/land cover change around Bolgoda wetland, Sri Lanka AHLCM Athapaththu *, Deepthi Wickramasinghe and MGMC Somachandra Department of Zoology and Environmental Sciences, Faculty of Science, University of Colombo, Colombo. Submitted: 15 August 2019; Revised: 21 January 2020; Accepted: 23 March 2020 Abstract: Wetlands are among the most productive ecosystems habitats are particularly important for livelihood of the in the world. Urban wetlands are increasingly aff ected by communities who live in the area. For instance, fi sheries population growth and developmental activities. A buff er and tourism are dependent on wetlands (Gachhadar region of 60 m from the Bolgoda lake boundary was gazetted et al ., 2004). Ecosystem services provided by wetlands as an Environmental Protection Area (EPA) by the Central include absorption of pollutants, carbon sequestration, Environmental Authority in 2009 as it is the largest freshwater wetland in Sri Lanka. This study attempts to quantify the land groundwater recharge, and disaster mitigation (Chmura use changes during the period of 2001 to 2019 using GIS and et al ., 2003; Chen et al ., 2008; Ramsar Convention to identify the ‘hot spots’ where a signifi cant change in land use Secretariat, 2014). Yet human induced land use/land occurred. Land use/land cover (LULC) changed in the Bolgoda cover (LULC) changes aff ect the wetlands worldwide wetland area disproportionately. The greatest proportion of as well as services provided by them (Zhao et al ., 2004; loss of natural area was observed in dense vegetation, where a Zorrilla-Miras et al ., 2014). -

WEPA Outlook on Water Environmental Management in Asia 2012
Ministry of the Environment WEPA Outlook on 2012 Water Environmental Management WEPA Outlook on Water Environmental Management in Asia 2012 Water Environment Partnership in Asia (WEPA) Ministry of the Environment, Japan Institute for Global Environmental Strategies (IGES) Outlook on Water Environmental Management in Asia 2012 Copyright © 2012 Ministry of the Environment, Japan. All rights reserved. No parts of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without prior permission in writing from Ministry of the Environment Japan through the Institute for Global Environment Strategies (IGES), which serves as the WEPA Secretariat. ISBN: 978-4-88788-108-2 This publication is made as a part of WEPA and published by the Institute for Global Environmental Strategies (IGES). Although every effort is made to ensure objectivity and balance, the publication of study results does not imply WEPA partner country’s endorsement or acquiescence with its conclusions. Ministry of the Environment, Japan 1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo, 100-8795, Japan Tel: +81-(0)3-3581-3351 http://www.env.go.jp/en/ Institute for Global Environmental Strategies (IGES) 2108-11 Kamiyamaguchi, Hayama, Kanagawa, 240-0115, Japan Tel: +81-(0)46-855-3700 http://www.iges.or.jp/ The research team for WEPA Outlook 2012 includes the following IGES members: [Drafting team] Yatsuka Kataoka, Director, Freshwater Sub-group Tetsuo Kuyama, -

Integrated Strategic Environmental Assessment of the Northern Province of Sri Lanka Report
Integrated Strategic Environmental Assessment of the Northern Province of Sri Lanka A multi-agency approach coordinated by Central Environment Authority and Disaster Management Centre, Supported by United Nations Development Programme and United Nations Environment Programme Integrated Strategic Environmental Assessment of the Northern Province of Sri Lanka November 2014 A Multi-agency approach coordinated by the Central Environmental Authority (CEA) of the Ministry of Environment and Renewable Energy and Disaster Management Centre (DMC) of the Ministry of Disaster Management, supported by United Nations Development Programme (UNDP) and United Nations Environment Programme (UNEP) Integrated Strategic Environment Assessment of the Northern Province of Sri Lanka ISBN number: 978-955-9012-55-9 First edition: November 2014 © Editors: Dr. Ananda Mallawatantri Prof. Buddhi Marambe Dr. Connor Skehan Published by: Central Environment Authority 104, Parisara Piyasa, Battaramulla Sri Lanka Disaster Management Centre No 2, Vidya Mawatha, Colombo 7 Sri Lanka Related publication: Map Atlas: ISEA-North ii Message from the Hon. Minister of Environment and Renewable Energy Strategic Environmental Assessment (SEA) is a systematic decision support process, aiming to ensure that due consideration is given to environmental and other sustainability aspects during the development of plans, policies and programmes. SEA is widely used in many countries as an aid to strategic decision making. In May 2006, the Cabinet of Ministers approved a Cabinet of Memorandum -

The Effect of Water Bodies As a Determinant Force in Generating Urban Form
The Effect of Water Bodies as a Determinant Force in Generating Urban Form. A Study on Creating a Symbiosis between the two with a case study of the Beira Lake, City of Colombo. K. Pradeep S. S. Fernando. 139404R Degree of Masters in Urban Design 2016 Department of Architecture University of Moratuwa Sri Lanka The Effect of Water Bodies as a Determinant Force in Generating Urban Form. A Study on Creating a Symbiosis between the two with a case study of the Beira Lake, City of Colombo. K. Pradeep S. S. Fernando. 139404R Degree of Masters in Urban Design 2016 Department of Architecture University of Moratuwa Sri Lanka THE EFFECT OF WATER BODIES AS A DETERMINANT FORCE IN GENERATING URBAN FORM - WITH A STUDY ON CREATING A SYMBIOSIS BETWEEN THE TWO WITH A CASE STUDY OF THE BEIRA LAKE, CITY OF COLOMBO. Water bodies present in Urban Contexts has been a primary determinant force in the urban formation and settlement patterns. With the evolutionary patterns governing the cities, the presence of water bodies has been a primary generator bias, thus being a primary contributor to the character of the city and the urban morphology. Urban form can be perceived as the pattern in which the city is formed where the street patterns and nodes are created, and the 03 dimensional built forms, which holistically forms the urban landscape. The perception of urban form has also been a key factor in the human response to the built massing, and fabric whereby the activity pattern is derived, with the sociological implications. DECLARATION I declare that this my own work and this dissertation does not incorporate without acknowledgment any material previously submitted for a Degree or Diploma in any University or any Institute of Higher Learning and to the best of my knowledge and belief it does not contain any materials previously published or written by another person except where acknowledgement is made in the text. -
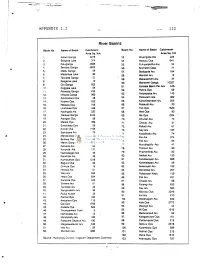
River Basins
APPENDIX I.I 122 River Basins Basin No Name of Basin Catchment Basin No. Name of Basin Catchment Area Sq. Km. Area Sq. Km 1. Kelani Ganga 2278 53. Miyangolla Ela 225 2. Bolgoda Lake 374 54. Maduru Oya 1541 3. Kaluganga 2688 55. Pulliyanpotha Aru 52 4. Bemota Ganga 6622 56. Kirimechi Odai 77 5. Madu Ganga 59 57. Bodigoda Aru 164 6. Madampe Lake 90 58. Mandan Aru 13 7. Telwatte Ganga 51 59. Makarachchi Aru 37 8. Ratgama Lake 10 60. Mahaweli Ganga 10327 9. Gin Ganga 922 61. Kantalai Basin Per Ara 445- 10. Koggala Lake 64 62. Panna Oya 69 11. Polwatta Ganga 233 12. Nilwala Ganga 960 63. Palampotta Aru 143 13. Sinimodara Oya 38 64. Pankulam Ara 382 14. Kirama Oya 223 65. Kanchikamban Aru 205 15. Rekawa Oya 755 66. Palakutti A/u 20 16. Uruhokke Oya 348 67. Yan Oya 1520 17. Kachigala Ara 220 68. Mee Oya 90 18. Walawe Ganga 2442 69. Ma Oya 1024 19. Karagan Oya 58 70. Churian A/u 74 20. Malala Oya 399 71. Chavar Aru 31 21. Embilikala Oya 59 72. Palladi Aru 61 22. Kirindi Oya 1165 73. Nay Ara 187 23. Bambawe Ara 79 74. Kodalikallu Aru 74 24. Mahasilawa Oya 13 75. Per Ara 374 25. Butawa Oya 38 76. Pali Aru 84 26. Menik Ganga 1272 27. Katupila Aru 86 77. Muruthapilly Aru 41 28. Kuranda Ara 131 78. Thoravi! Aru 90 29. Namadagas Ara 46 79. Piramenthal Aru 82 30. Karambe Ara 46 80. Nethali Aru 120 31.