A Case of Long QT Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ever 2018 Eupo 2018
European Association for Vision and Eye Research European University Professors of Ophthalmology EVER 2018 Annual Congress October 4-6, 2018 EUPO 2018 Course on Retina, Intraocular Inflammation & Uveitis October 3-4, 2018 Programme book Nice, France www.ever.be www.eupo.eu European Association for Vision and Eye Research EVER 20October 17-1919 in Nice, France www.ever.be 1 Table of contents Word from the president ....................................................................................................................................2 About EVER ..........................................................................................................................................................3 EVER Membership ...............................................................................................................................................4 Speakers’ affiliation to scientific sections .........................................................................................................5 Composition of the board 2018 .........................................................................................................................8 Venue ................................................................................................................................................................... 10 Congress information ....................................................................................................................................... 11 Programme information ....................................................................................................................................15 -

Short-Term Rapamycin Persistently Improves Cardiac Function After Cessation of Treatment in Aged Male and Female Mice
Short-term rapamycin persistently improves cardiac function after cessation of treatment in aged male and female mice. Ellen Quarles A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2017 Reading Committee: Peter Rabinovitch, Chair Michael MacCoss David Marcinek Program Authorized to Offer Degree: Pathology © Copyright 2017 Ellen Quarles University of Washington Abstract Short-term rapamycin persistently improves cardiac function after cessation of treatment in aged male and female mice. Ellen Quarles Chair of the Supervisory Committee: Peter Rabinovitch, Professor and Vice Chair of Research Department of Pathology Cardiac aging is an intrinsic process that results in impaired cardiac function and dysregulation of cellular and molecular quality control mechanisms. These effects are evident in the decline of diastolic function, increase in left ventricular hypertrophy, metabolic substrate shifts, and alterations to the cardiac proteome. This thesis covers the quality control mechanisms that are associated with cardiac aging, results from an anti-aging intervention in aged mice, and a review of mitochondrial dysfunction in the heart. Chapter one is a review of the quality control mechanisms in aging myocardium. Chapter two consists of the results of several mouse experiments that compare the cardiac function, proteomes, and metabolomes of aged and young controls, along with rapamycin treated aged mice. The novelty of this study comes from the inclusion of a group of animals treated only transiently with the drug, then followed for eight weeks post-drug-removal. This persistence cohort may hold clues to deriving long-lasting benefits of rapamycin with only transient treatment. -

Postural Heart Block*
Br Heart J: first published as 10.1136/hrt.44.2.221 on 1 August 1980. Downloaded from Case reports Br Heart J 1980; 44: 221-3 Postural heart block* PETER E SEDA, JOHN H McANULTY, C JOE ANDERSON From the Department of Medicine, University of Oregon Health Sciences Center, Portland, Oregon, USA SUMMARY A patient presented with orthostatic dizziness and syncope caused by postural heart block. When the patient was supine, atrioventricular conduction was normal and he was asymptomatic; when he was standing he developed second degree type II block and symptoms. The left bundle-branch block on his electrocardiogram and intracardiac electrophysiological study findings suggest that this heart block occurred distal to the His bundle. Orthostatic symptoms are usually presumed to be secondary to an inappropriate distribution of intravascular volume or to autonomic nervous system abnormalities. As shown in this patient, these symptoms may be the result of orthostatic heart block. Ambulatory monitoring may be useful in patients with orthostatic neurological symptoms, particularly when conduction abnormalities are present on the electrocardiogram. Orthostatic neurological symptoms usually result minute and regular, and increased to 90 beats a from inadequate cerebral perfusion caused by minute with some irregularity when he was upright. disturbances of the autonomic nervous system,'-3 The carotid pulse was normal, and there were no ineffective or inappropriate shifts in volume carotid bruits. The cardiac impulse was normal. http://heart.bmj.com/ distribution,4 or drugs.5 We report a patient with The second heart sound was paradoxically split. orthostatic dizziness and syncope caused by inter- There was a grade 2/6 apical systolic murmur. -

The Frequency of Rhythm and Conduction Abnormalities and Benefits of 24-Hour Holter Electrocardiogram on Detecting These Abnormalities
ORIGINAL ARTICLE East J Med 24(3): 303-309, 2019 DOI: 10.5505/ejm.2019.31932 The Frequency of Rhythm and Conduction Abnormalities and Benefits of 24-Hour Holter Electrocardiogram on Detecting These Abnormalities In Patients With Acute Rheumatic Fever Serdar Epçaçan*, Yasemin Nuran Dönmez University of Health Sciences, Van Training and Research Hospital, Department of Pediatric Cardiology, Van, Turkey ABSTRACT During the acute phase of acute rheumatic fever (ARF), cardiac arrhythmias and conduction disorders may occur. Standard electrocardiogram (ECG) may be insufficient in the cases of possible paroxysmal rhythm or conduction abnormalities. The aim of this study is to evaluate arrhythmias and conduction disorders and benefits of 24-hour Holter ECG on detecting these disorders in children with ARF. Two hundred and ten patients who were diagnosed with ARF during a four-year period, were retrospectively analyzed. Demographic characteristics, clinical, laboratory, and echocardiographic findings of the patients were evaluated. Standard ECG and 24-hour Holter analysis were examined. First (47.8%), second (6.9%) and third degree (4.3%) atrioventricular (AV) blocks, bundle branch blocks (9.8%), intermittent pre-excitation (1.1%), accelerated nodal rhythm (15.2%), supraventricular (10.9%) and ventricular premature contractions (8.7%), as well as supraventricular (3.3%) and ventricular tachycardia (1.1%) were detected with 24 -hour Holter ECG. Frequency of both rhythm and conduction abnormalities were detected higher with Holter ECG than 12-lead ECG, and this was statistically significant (p<0.05). Second degree type II AV block and non-sustained supraventricular tachycardia as well as intermittent complete AV block were detected on 24-hour Holter analysis in patients with normal initial standard ECG. -

Answer: E) Atrial Fibrillation with Complete Heart Block Teaching Point
Answer: e) Atrial fibrillation with complete heart block Teaching Point: A slow regular ventricular rate in a patient with concurrent atrial fibrillation, as seen in this ECG, is diagnostic of complete heart block. Atrial fibrillation creates a diagnostic dilemma for identifying AV nodal disease or block. Close scrutiny should be placed on R-R intervals to identify patterns or regularity (1). Clinicians should be wary of a regular heart rate in a patient with persistent atrial fibrillation, especially in those using digitalis. If an AV nodal block is identified, it may be transient, and a search for reversible causes is indicated as in all cases of complete heart block prior to pacemaker placement. Electrolyte abnormalities, ischemia, and medications remain the leading reversible causes (2,3). The patient in this case was transferred to the Emergency Department and admitted for further observation. Ischemia was ruled out. Carvedilol was held, and he was diuresed. He continued to demonstrate adequate chronotropic response with exertion. The complete heart block soon resolved, and he was diuresed to euvolemia. Pacemaker placement was deferred given the transient nature of the AV block in the context of recent beta-blocker usage. He was discharged home with continuous heart rhythm monitoring without any further evidence of complete heart block. References: 1. Urbach JR, Grauman JJ, Straus SH. Quantitative Methods for the Recognition of Atrioventricular Junctional Rhythms in Atrial Fibrillation. Circulation. 1969; 39: 803- 817. 2. Kojic EM, Hardarson T, Sigfusson N, Sigvaldason H. The prevalence and prognosis of third-degree atrioventricular conduction block: the Reykjavik study. J Intern Med. -

Cardiac Pacing in Incomplete Atrioventricular Block with Atrial Fibrillation
Br Heart J: first published as 10.1136/hrt.35.11.1154 on 1 November 1973. Downloaded from British Heart journal, I973, 35, I154-1I60. Cardiac pacing in incomplete atrioventricular block with atrial fibrillation D. S. Reid, S. J. Jachuck, and C. B. Henderson From the Department of Cardiology, Newcastle General Hospital, Newcastle upon Tyne Three cases with a slow irregular ventricular response to atrialfibrillation, who benefitedfrom cardiac pacing, are described; two had ischaemic heart disease, and one had cardiomyopathy. In thefirst case the slow ventricu- lar response to atrialfibrillation was a result of incomplete atrioventricular nodal block, and in the other two His bundle electrograms demonstrated that the slow ventricular response was due to bilateral bundle-branch block. The association of atrial fibrillation and conduction delays in the atrioventricular node and bundle- branches is discussed. The value of His bundle recordings in the investigation of these cases is shown and the importance of cardiac pacing in treatment is stressed. Cardiac pacing is now a generally accepted method hypotension. Intravenous atropine given before transfer of treatment in patients with complete heart block or had resulted in a paroxysm of ventricular tachycardia. bilateral bundle-branch block who have Adam- On admission there was no evidence of cardiac failure Stokes attacks or a low output to and the blood pressure was I05/50 mmHg. An electro- syndrome leading cardiogram showed atrial fibrillation with an irregular angina or cardiac failure. cardiac is However, pacing ventricular response of 40 to 44 beats a minute and acute now also becoming more widely used in the treat- inferolateral myocardial infarction (Fig. -
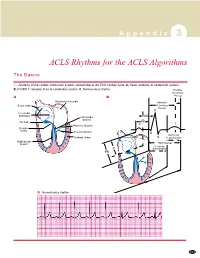
ACLS Rhythms for the ACLS Algorithms
A p p e n d i x 3 ACLS Rhythms for the ACLS Algorithms The Basics 1. Anatomy of the cardiac conduction system: relationship to the ECG cardiac cycle. A, Heart: anatomy of conduction system. B, P-QRS-T complex: lines to conduction system. C, Normal sinus rhythm. Relative Refractory A B Period Bachmann’s bundle Absolute Sinus node Refractory Period R Internodal pathways Left bundle AVN branch AV node PR T Posterior division P Bundle of His Anterior division Q Ventricular Purkinje fibers S Repolarization Right bundle branch QT Interval Ventricular P Depolarization PR C Normal sinus rhythm 253 A p p e n d i x 3 The Cardiac Arrest Rhythms 2. Ventricular Fibrillation/Pulseless Ventricular Tachycardia Pathophysiology ■ Ventricles consist of areas of normal myocardium alternating with areas of ischemic, injured, or infarcted myocardium, leading to chaotic pattern of ventricular depolarization Defining Criteria per ECG ■ Rate/QRS complex: unable to determine; no recognizable P, QRS, or T waves ■ Rhythm: indeterminate; pattern of sharp up (peak) and down (trough) deflections ■ Amplitude: measured from peak-to-trough; often used subjectively to describe VF as fine (peak-to- trough 2 to <5 mm), medium-moderate (5 to <10 mm), coarse (10 to <15 mm), very coarse (>15 mm) Clinical Manifestations ■ Pulse disappears with onset of VF ■ Collapse, unconsciousness ■ Agonal breaths ➔ apnea in <5 min ■ Onset of reversible death Common Etiologies ■ Acute coronary syndromes leading to ischemic areas of myocardium ■ Stable-to-unstable VT, untreated ■ PVCs with -

Splicing Repression Is a Major Function of Tdp-43 in Motor Neurons
SPLICING REPRESSION IS A MAJOR FUNCTION OF TDP-43 IN MOTOR NEURONS: THERAPEUTIC IMPLICATIONS FOR AMYOTROPHIC LATERAL SCLEROSIS by Aneesh N. Donde A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland May, 2018 Title: Splicing Repression is a Major Function of Tdp-43 in Motor Neurons Ph.D Dissertator: Aneesh N. Donde Ph.D Advisor: Philip C. Wong, Ph.D Abstract Nuclear depletion of TDP-43, an RNA binding protein which serves to protect the transcriptome by repressing aberrant splicing, may underlie neurodegeneration in amyotrophic lateral sclerosis (ALS). As multiple functions have been ascribed to TDP-43, whether splicing repression is its major role in motor neurons – that may be compromised in ALS – remains to be established. Here, we show that TDP-43 mediated splicing repression is central to the physiology of motor neurons. To validate TDP-43 mediated splicing repression as a therapeutic target, an AAV9-mediated gene delivery approach was employed to deliver a chimeric protein comprised of the N-terminal RNA recognition domain of TDP-43 fused to an unrelated splicing repressor (RAVER1) to mice lacking TDP-43 in motor neurons. This strategy allowed long-term expression of the repressor without any untoward effects, delayed the onset and slowed the progression of disease, and extended survival. In treated mice, evidence of aberrant splicing was markedly decreased and accompanied by amelioration of motor neuron loss. These findings establish that splicing repression is a principal role of TDP-43 in motor neurons and support the idea that loss of TDP-43-mediated splicing repression represents a key pathogenic mechanism underling motor neuron loss, validating a novel mechanism-based therapeutic strategy for ALS. -

South Australian Government Boards and Committees Information As at 30 June 2020
OFFICIAL South Australian Government Boards and Committees Information As at 30 June 2020 OFFICIAL OFFICIAL South Australian Government Boards and Committees As at 30 June 2020 Introduction This is the 24th annual report to Parliament of consolidated South Australian Government board and committee information1. The report sets out the membership and remuneration arrangements of 196 part-time government boards and committees as at 30 June 2020 in order of ministerial portfolio. The information has been sourced from the Boards and Committees Information System (BCIS), a database administered by the Department of the Premier and Cabinet, following extensive consultation with all ministerial offices and stakeholder agencies. Definition of boards and committees in the report The boards and committees included in this report are those which are: • established by or under an Act of Parliament of South Australia (generally excluding the Local Government Act 1999) and have a majority of members appointed by either a minister or the Governor; or • established by a minister or legal instrument such as a constitution or charter, have a majority of members appointed by a minister, and have at least one member in receipt of remuneration. The report should not be considered to be a complete listing of all government boards and committees. 1 Note: The 2019 report, which was the 23rd such report, was incorrectly published as the 22nd report. This error dates to the 2014 annual report, which was incorrectly published as the 17th report, resulting in all subsequent reports being incorrectly labelled. This error has been corrected in the copies of the report available on the DPC website Page 2 of 9 OFFICIAL OFFICIAL Highlights Number of boards and committees 196 boards and committees are identified in the 2020 report. -

In Re Network Associates, Inc. Securities Litigation 00-CV-4849
MC66N Rejected or Ineligible Claimants Page 1 of 251 MC66N138 NETWORK ASSOCIATES, INC. II SECUR REPS 13-Jun-05 11:50 AM Reason Deemed Claim Number Name City State Ineligible 10559 WATKINS, JOYCE E ATLANTA GA FATAL LINE 12498 1199 HEALTH CARE EMP CHICAGO IL NO LOSS 7784 16105 PIMCO IARORCIH NEW YORK NY NO LOSS 2259 20 UIT FUNDS NEWYORK NY INTRINSICALLY INELIGIBLE 2109830 33 WEST CLINTON AVEN TOMS RIVER NJ NO LOSS 9826 3M ERIP TRUST PITTSBURGH PA FATAL LINE 8466 4 Z'S INVESTMENTS PA WATERFORD MI NO LOSS 1080 45 INDIANA HOSPITOL PHILA PA NO LOSS 8779 777-H F II 1990 STLM DETROIT MI NO LOSS 2093721 800 PRE-EMPTION ROAD GENEVA NY NO LOSS 2247 91325 CANADA INC NO LOSS 8375 A DIAMOND FAMILY TRU LOS ANGELES CA NO LOSS 2065510 A F & N CIRAMELLA RE DAYTON OH NO LOSS 2029983 A R W SUPPORT TRUST TAYLORVILLE IL INTRINSICALLY INELIGIBLE 12103 AAA MI RESTRUCTURING CHICAGO IL NO LOSS 2093159 AAOF ENDOWMENT FUND SAINT LOUIS MO NO LOSS 2095827 AARON, BRIAN & CAROL BETHESDA MD FATAL LINE 2091134 ABAD, MARIO & NAVATA WEST ORANGE NJ NO LOSS 2027060 ABBAMONTE, MICHAEL & SOLON OH INTRINSICALLY INELIGIBLE 2101711 ABBETT, DEANNA L SAN JOSE CA FATAL LINE 2055264 ABDELQADER, MAHMUD S COLLINSVILLE IL NO LOSS 2030513 ABEGGLEN, CHERYL ROS CHADRON NE INTRINSICALLY INELIGIBLE 4336 ABEL, DAVID MELVILLE NY NO LOSS 2015464 ABEL, KEVIN KEW GARDENS NY NO LOSS 2020708 ABEL, STEPHEN ONEIDA NY NO LOSS 8542 ABEUTT, BETWORT MERRICK NY NO LOSS 2127049 ABLEY, PETER & VIBEK NO LOSS 6756 ABN AMRO N QUINCY MA INTRINSICALLY INELIGIBLE 4204 ABN AMRO SMALL CAP F CHICAGO IL NO LOSS 1214 -
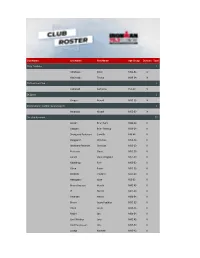
Club Name Last Name First Name Age Group Division Total 3City
Club Name Last Name First Name Age Group Division Total 3City Triathlon 2 Adolfsson Peter M50-54 IV Hekimoglu Tayfun M30-34 IV 3D Triathlon Club 1 Campbell Caitriona F55-59 V 3K Sport 1 Gregec Nenad M35-39 IV 338 Småland Triathlon & Multisport 1 Bergqvist Mikael M55-59 IV /tri club denmark 37 Jensen Brian Nors M40-44 II Ottosen Brian Peldrup M30-34 II Skovgaard Pedersen Camilla F40-44 II Daugaard Christian M50-54 II Wolthers-Petersen Christian M35-39 II Petersen Claus M55-59 II Larsen Claus Wiegand M55-59 II Moeldrup Finn M55-59 II Olsen Frank M55-59 II Ellekilde Frederik M25-29 II Hebsgaard Gitte F55-59 II Bruun Axelsen Henrik M45-49 II If Henrik M65-69 II Petersen Henrik M50-54 II Bruun Jacob Fuglkjær M35-39 II Olsen Jacob M30-34 II Reichl Jan M50-54 II Lind-Winther Jens M45-49 II Hvid Rasmussen Jim M55-59 II Luxhøi Kenneth M45-49 II Fischer Lars M45-49 II Vogelius Lasse M30-34 II Kjær Olesen Malene F30-34 II Vergroesen Marcus M30-34 II Andersen Martin M40-44 II Orby Martin Falck M30-34 II Mortensen Mathias Loft M25-29 II Andersen Mathias Meier M25-29 II Rolsted Michael M45-49 II Haakonsen Nick M40-44 II Jakobsen Nikolaj M25-29 II skjoldemose nikolaj M30-34 II Rosenlund Nino M45-49 II Larsen Peter M55-59 II Pauly Rune M45-49 II Kousgaard Sune M25-29 II Gammelvind Thomas M40-44 II Aalborg Triathlonklub 1 Kvist-Ibsen Anna F18-24 V Aegir 3 13 Jónsdóttir Dagný F30-34 V Jónsdóttir Erna Hlif F40-44 V Thorarinsson Finnbogi M55-59 V Jónsdóttir Guðrún F45-49 V Karlsdottir Inga Dagmar F45-49 V Poulsen Ingi M40-44 V Jónsson Jón Orri M25-29 V Steinadottir Kristin L. -

The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-Induced Liver Injuries
The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-induced Liver Injuries By Jessica A. Williams Submitted to the graduate degree program in Pharmacology, Toxicology, and Therapeutics and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Dr. Wen-Xing Ding, PhD ________________________________ Dr. Hartmut Jaeschke, PhD ________________________________ Dr. Michele Pritchard, PhD ________________________________ Dr. Udayan Apte, PhD ________________________________ Dr. Benyi Li, MD, PhD Date Defended: May 28th, 2015 The Dissertation Committee for Jessica A. Williams certifies that this is the approved version of the following dissertation: The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-induced Liver Injuries ________________________________ Chairperson Dr. Wen-Xing Ding Date approved: June 8th, 2015 ii Abstract Acetaminophen (APAP) is the leading cause of acute liver failure in the United States, and alcoholic liver disease (ALD) is a worldwide health problem that claims two million lives per year. Currently, the only cure for either disease is liver transplantation in severe disease states. Therefore, new therapeutic options for treatment of these liver diseases are greatly needed. To develop new therapeutic options, the mechanisms involved in APAP and alcohol-induced liver toxicities must be better understood. We previously demonstrated that autophagy was protective against both APAP and alcohol-induced liver injuries by removing damaged mitochondria by mitophagy, which is a selective form of autophagy specific for mitochondria. However, the mechanisms for induction of mitophagy in the liver are unknown. Parkin is an E3 ubiquitin ligase that is well known to be required for mitophagy induction in mammalian cell models after mitochondrial depolarization.