(Middle Jurassic): a Key Interval in the Early Mesozoic Phytoplankton Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Jurassic Pleurotomarioidean Gastropod Laevitomaria and Its Palaeobiogeographical History
The Jurassic pleurotomarioidean gastropod Laevitomaria and its palaeobiogeographical history ROBERTO GATTO, STEFANO MONARI, JÁNOS SZABÓ, and MARIA ALESSANDRA CONTI Gatto, R., Monari, S., Szabó, J., and Conti, M.A. 2015. The Jurassic pleurotomarioidean gastropod Laevitomaria and its palaeobiogeographical history. Acta Palaeontologica Polonica 60 (1): 217–233. The genus Laevitomaria is reviewed and its palaeobiogeographical history is reconstructed based on the re-examination of its type species L. problematica, the study of material stored at the National Natural History Museum of Luxembourg, and an extensive review of the literature. The systematic study allows ascribing to Laevitomaria a number of Jurassic species from the western European region formerly included in other pleurotomariid genera. The following new combi- nations are proposed: Laevitomaria allionta, L. amyntas, L. angulba, L. asurai, L. daityai, L. fasciata, L. gyroplata, L. isarensis, L. joannis, L. repeliniana, L. stoddarti, L. subplatyspira, and L. zonata. The genus, which was once considered as endemic of the central part of the western Tethys, shows an evolutionary and palaeogeographical history consider- ably more complex than previously assumed. It first appeared in the Late Sinemurian in the northern belt of the central western Tethys involved in the Neotethyan rifting, where it experienced a first radiation followed by an abrupt decline of diversity in the Toarcian. Species diversity increased again during Toarcian–Aalenian times in the southernmost part of western European shelf and a major radiation occurred during the Middle Aalenian to Early Bajocian in the northern Paris Basin and southern England. After a latest Bajocian collapse of diversity, Laevitomaria disappeared from both the central part of western Tethys and the European shelf. -
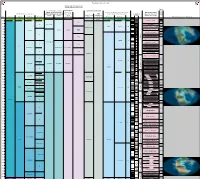
Expanded Jurassic Timescale
TimeScale Creator 2012 chart Russian and Ural regional units Russia Platform regional units Calca Jur-Cret boundary regional Russia Platform East Asian regional units reous stages - British and Boreal Stages (Jur- Australia and New Zealand regional units Marine Macrofossils Nann Standard Chronostratigraphy British regional Boreal regional Cret, Perm- Japan New Zealand Chronostratigraphy Geomagnetic (Mesozoic-Paleozoic) ofossil stages stages Carb & South China (Neogene & Polarity Tethyan Ammonoids s Ma Period Epoch Age/Stage Substage Cambrian) stages Cret) NZ Series NZ Stages Global Reconstructions (R. Blakey) Ryazanian Ryazanian Ryazanian [ no stages M17 CC2 Cretaceous Early Berriasian E Kochian Taitai Um designated ] M18 CC1 145 Berriasella jacobi M19 NJT1 Late M20 7b 146 Lt Portlandian M21 Durangites NJT1 M22 7a 147 Oteke Puaroan Op M22A Micracanthoceras microcanthum NJT1 Penglaizhenian M23 6b 148 Micracanthoceras ponti / Volgian Volgian Middle M24 Burckhardticeras peroni NJT1 Tithonian M24A 6a 149 M24B Semiformiceras fallauxi NJT15 M25 b E 150 M25A Semiformiceras semiforme NJT1 5a Early M26 Semiformiceras darwini 151 lt-Oxf N M-Sequence Hybonoticeras hybonotum 152 Ohauan Ko lt-Oxf R Kimmeridgian Hybonoticeras beckeri 153 m- Lt Late Oxf N Aulacostephanus eudoxus 154 m- NJT14 Late Aspidoceras acanthicum Oxf R Kimmeridgian Kimmeridgian Kimmeridgian Crussoliceras divisum 155 155.431 Card- N Ataxioceras hypselocyclum 156 E Early e-Oxf Sutneria platynota R Idoceras planula Suiningian 157 Cal- Oxf N Epipeltoceras bimammatum 158 lt- Lt Callo -

A Key Interval in the Early Mesozoic Phytoplankton Radiation
1 The Bajocian (Middle Jurassic): a key interval in the early Mesozoic phytoplankton radiation 2 3 Nickolas J. Wiggan a.b*, James B. Riding b, Robert A. Fensome c, Emanuela Mattioli d 4 5 Insert addresses here 6 7 ABSTRACT 8 Dinoflagellates and coccolithophores are two of the most important groups of phytoplankton 9 in the modern oceans. These groups originated in the Triassic and radiated through the early 10 Mesozoic, rising to ecological prominence. Within this long-term radiation, important short- 11 term intervals of evolutionary and ecological change can be recognised. The Bajocian 12 (Middle Jurassic, ~170–168 Ma) was characterised by an important ecological transition 13 within the coccolithophores, and the radiation of one of the principal families of cyst-forming 14 dinoflagellates, the Gonyaulacaceae. During the Early Bajocian, the coccolith genus 15 Watznaueria diversified and expanded ecologically to dominate coccolith floras, a situation 16 which continued for the remainder of the Mesozoic. This pattern was paralleled within 17 dinoflagellate cyst floras by the ecological dominance of the genus Dissiliodinium in the mid- 18 palaeolatitudes. These phenomena appear to be linked to a positive carbon isotope shift, and 19 an interval of enhanced productivity driven by a shift to a more humid climate, enhanced 20 continental weathering and nutrient flux. The latest Early Bajocian to earliest Bathonian was 21 then characterised by the rapid increase in diversity of dinoflagellate cysts within the family 22 Gonyaulacaceae. Through this interval, the Gonyaulacaceae transitioned from being a 23 relatively minor component of dinoflagellate cyst floras, to becoming one of the prominent 24 groups of cyst-forming dinoflagellates, which has persisted to the Holocene. -

Integrated Biostratigraphy Across the Aalenian/Bajocian Boundary of the Central High Atlas, Morocco
Volumina Jurassica, 2015, Xiii (1): 27–42 Doi: 10.5604/17313708 .1148554 Integrated biostratigraphy across the Aalenian/Bajocian boundary of the Central High Atlas, Morocco Driss SADKI 1 Key words: Aalenian/Bajocian boundary, Central High Atlas, Morocco, integrated biostratigraphy. Abstract. This work presents an integrated biostratigraphic study across the Aalenian/Bajocian boundary of the Central High Atlas of Morocco. The data that are the basis for this study were obtained from the region of Rich that is located in the center of the basin of the Moroccan High Atlas. This region presents a thick Aalenian–Bajocian succession with continuous marine sedimentation, which offers a rich and varied fauna whose analysis enables the compilation of an integrated biostratigraphy based on the different paleontological groups: ammonites, belemnites, brachiopods, bivalves, gastropods, calcareous nanno- fossils, foraminifera and ostracods. INTRODUCTION AMMONITES The Rich area, situated in the center of the basin of High The succession of ammonite faunas in the interval across Moroccan Atlas (Fig. 1), presents a thick Aalenian–Bajocian the Aalenian/Bajocian boundary, shows (Fig. 2) six succes- succession with continuous marine sedimentation, which of- sive assemblages (Sadki, Elmi, 1991), sometimes dominated fers rich and varied fossils. This section was proposed as the by the NW European graphoceratids, sometimes by Mediter- auxiliary stratotype of the Aalenian/Bajocian boundary in the ranean Province taxa. Furthermore the abundance of the Mediterranean Province (Sadki, 1994). An integrated bio- phylloceratids is similar to that of the hammatoceratids, and stratigraphic analysis of this section is presented here, detail- therefore shows the same trend as that of Mediterranean ing the distribution of the main paleontological groups: am- Province taxa. -

Paléontologie Au Luxembourg (2) A
Paléontologie au Luxembourg (2) A. Di Cencio, D. Sadki, R. Weis (eds.) R. Weis D. Sadki, A. Di Cencio, Les ammonites de la Minette Andrea Di Cencio, Driss Sadki, Paléontologie au Luxembourg (2) Luxembourg au Paléontologie Robert Weis (eds.) Ferrantia Travaux scientifiques du Musée national d'histoire naturelle Luxembourg www.mnhn.lu 83 2020 Ferrantia 83 2020 2020 83 Ferrantia est une revue publiée à intervalles non réguliers par le Musée national d’histoire naturelle à Luxembourg. Elle fait suite, avec la même tomaison, aux T M ’ L parus entre 1981 et 1999. Comité de rédaction: Eric Buttini Guy Colling Alain Frantz Thierry Helminger Ben Thuy Mise en page: Romain Bei Design: Thierry Helminger Prix du volume: 20 € Rédaction: Échange: Musée national d’histoire naturelle Exchange MnhnL Rédaction Ferrantia c/o Musée national d’histoire naturelle 25, rue Münster 25, rue Münster L-2160 Luxembourg L-2160 Luxembourg Tél +352 46 22 33 - 1 Tél +352 46 22 33 - 1 Fax +352 46 38 48 Fax +352 46 38 48 Internet: http://www.mnhn.lu/ferrantia/ Internet: http://www.mnhnl.lu/biblio/exchange email: [email protected] email: [email protected] Page de couverture: Bredyia subinsignis (Oppel, 1856), DOU833. Natural History Museum of Luxembourg. Citation: Di Cencio A., Sadki D., Weis R. (eds.) 2020. - Paléontologie au Luxembourg (2) - Les ammonites de la Minette. Ferrantia 83, Musée national d’histoire naturelle, Luxembourg, 129 p. Date de publication: 15 décembre 2020 (réception du manuscrit: 19 mai 2020) Impression: Imprimerie Centrale climatiquement neutre Impression | LU-319-JR8FDJV | www.natureOffice.com Ferrantia est publiée sous la licence Creative Commons BY-NC-ND 3.0 LU. -

A New Large−Bodied Theropod Dinosaur from the Middle Jurassic of Warwickshire, United Kingdom
A new large−bodied theropod dinosaur from the Middle Jurassic of Warwickshire, United Kingdom ROGER B.J. BENSON and JONATHAN D. RADLEY Benson, R.B.J. and Radley, J.D. 2010. A new large−bodied theropod dinosaur from the Middle Jurassic of Warwickshire, United Kingdom. Acta Palaeontologica Polonica 55 (1): 35–42. Previously undocumented postcranial material from the Chipping Norton Limestone Formation (Middle Jurassic: Lower Bathonian) of Cross Hands Quarry, near Little Compton, Warwickshire represents a new large−bodied theropod dinosaur, distinct from the contemporaneous Megalosaurus bucklandii. Cruxicheiros newmanorum gen. et sp. nov. is diagnosed by a single autapomorphy, the presence of a proximomedially inclined ridge within the groove that marks the lateral extent of the posterior flange of the femoral caput (trochanteric fossa). C. newmanorum shows three tetanuran features: widely separated cervical zygapophyses, a swollen ridge on the lateral surface of the iliac blade and an anterior spur of the caudal neural spines. However, due to fragmentary preservation its affinities within Tetanurae remain uncertain: phylogenetic analysis places it as the most basal tetanuran, the most basal megalosauroid (= spinosauroid) or the most basal neotetanuran. Key words: Dinosauria, Theropoda, Tetanurae, Megalosaurus, Cruxicheiros, Bathonian, Chipping Norton Limestone Formation, England. Roger B.J. Benson [[email protected]], Department of Earth Science, University of Cambridge, Downing Street, Cam− bridge, UK, CB2 3EQ; Jonathan D. Radley [[email protected]], Warwickshire Museum, Market Place, Warwick, UK CV34 4SA; School of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, UK B15 2TT. Received 8 July 2009, accepted 18 November 2009, available online 20 November 2009. -

The Graphoceratid Ammonite Succession in the Aalenian and Lowest Bajocian (Middle Jurassic) at Horn Park, Dorset, UK ROBERT B
The Graphoceratid Ammonite Succession in the Aalenian and lowest Bajocian (Middle Jurassic) at Horn Park, Dorset, UK ROBERT B. CHANDLER Riddlesdown High School, Purley, Surrey, CR8 lEX Summary In 1990 Callomon and Chandler set out a scheme of 16 faunal horizons for the Aalenian-Lower Bajocian of Dorset and Somerset based principally on graphoceratid ammonites. One locality, Horn Park, stands apart in displaying strata from almost all the horizons identified in that work. Yet little material from it has ever been figured. Here a section is given together with exhaustive tables of existing nominal morphospecies collected at each level and with figures that allow the variability of the suc- cessive, probably in most cases, monobiospecific assemblages to be assessed. A revised generic classification of the Graphoceratidae is presented. them precludes almost any satisfactory discussion of phyletic relationships and hence of the overall evolution The highly discontinuous, condensed and rapidly of the group. varying development of the Inferior Oolite in southern England was first perceived by S.S. Buckman (e.g. 1891, The biostratigraphy of the Graphoceratidae has now 1893, 1910) during his attempts to correlate sections by been rather well documented, for example by Rocha et means of ammonites. Leading amongst these in al. (1990), Henriques (1992; et al. 1994) in Portugal; the Aalenian part of the succession are the Linares and Sandoval (1990) in Spain, Sadki (1994) in Graphoceratidae, including such well known genera as Morocco. In Britain, Morton (1990; 1994) and Morton Leioceras, Ludwigia, Brasilia, Graphoceras and and Hudson (1995) have described the succession in Hyperlioceras. Scotland and compared it with Dorset (Morton and Chandler 1994). -

Jurassic (170 - 199 Ma Time-Slice) Time
Early Jurassic (170 - 199 Ma time-slice) Time ScaLe R Creator CHRONOS Cen Mesozoic Updated by James G. Ogg (Purdue University) and Gabi Ogg to: GEOLOGIC TIME SCALE 2004 (Gradstein, F.M., Ogg, J.G., Smith, A.G., et al., 2004) and The CONCISE GEOLOGIC TIME SCALE (Ogg, J.G., Ogg, G., and Gradstein, F.M., 2008) Paleozoic Sponsored, in part, by: Precambrian ICS Based, in part, on: CENOZOIC-MESOZOIC BIOCHRONOSTRATIGRAPHY: JAN HARDENBOL, JACQUES THIERRY, MARTIN B. FARLEY, THIERRY JACQUIN, PIERRE-CHARLES DE GRACIANSKY, AND PETER R. VAIL,1998. Mesozoic and Cenozoic Sequence Chronostratigraphic Framework of European Basins in: De Graciansky, P.- C., Hardenbol, J., Jacquin, Th., Vail, P. R., and Farley, M. B., eds.; Mesozoic and Cenozoic Sequence Stratigraphy of European Basins, SEPM Special Publication 60. Standard Geo- Ammonites Sequences Ammonites Sequences Smaller Benthic Foraminifers Larger Benthic Foraminifers Calcareous Nannofossils Dinoflagellate Cysts Radiolarians Belemnites Brachiopods Ostracodes Charophytes Stage Age Chronostratigraphy magnetic North Atlantic Tethys Age Polarity Sequences Boreal Sequences T-R Major T-R Period Epoch Stage Substage Boreal Boreal T-R Cycles Tethyan Global, Tethyan Cycles Cycles Zones Tethyan markers Zones Zonal Markers Zonal Markers Zones Boreal Zonal Markers Other Boreal Nannofossils Zones Zonal Markers Other Dinocysts Tethyan Dinocysts Zones Zonal Markers NW Europe Boreal Tethyan Boreal Ostracodes Tethyan Ostracodes Markers Lt. Bajoc. Strenoceras niortense Teloceras banksi Strenoceras niortense Teloceras banksi DSJ14 Lithodinia valensii Belemnopsis Lissajouthyris Lissajouthyris [un-named] 169.8 Stephanolithion speciosum Mancodinium semitabulatum, 169.8 L. galeata mg P., speciosum Durotrigia daveyi, Phallocysta apiciconus matisconensis matisconensis Glyptocythere scitula Teloc. blagdeni Bj3 Telo. blagdeni Bj3 NJ10 Andromeda depressa, G. -

1 the Literature on Triassic, Jurassic and Earliest Cretaceous Dinoflagellate Cysts: Supplement 4 2 3 James B
1 The literature on Triassic, Jurassic and earliest Cretaceous dinoflagellate cysts: supplement 4 2 3 James B. Riding 4 5 British Geological Survey, Environmental Science Centre, Keyworth, Nottingham NG12 6 5GG, United Kingdom 7 8 CONTACT James B. Riding email [email protected] 9 10 ABSTRACT 11 Since the publication of four compilations issued between 2012 and 2019, 93 further 12 published contributions on Triassic, Jurassic and earliest Cretaceous (Berriasian) 13 dinoflagellate cysts from Africa, North America, South America, the Arctic, Australasia, East 14 Europe, West Europe, the Middle East and Russia have been discovered in the literature, or 15 were issued in the last 12 months (i.e. between February 2018 and January 2019). Of these, 16 55 were published during 2018 and 2019, making this period a very productive one. These 17 studies are mostly on the Late Triassic and Early Jurassic of Europe. All the 93 items are 18 listed herein with digital object identifier (doi) numbers where available, as well as a 19 description of each item as a string of keywords. Publications on West Europe comprise 20 31.2% of the total, and items on Africa, the Arctic, Australasia, East Europe and Russia are 21 also significant (15.1%, 6.5%, 7.5%, 9.7% and 14.0% respectively). The least well- 22 represented regions are North America, South America and the Middle East (2.2%, 1.1% and 23 1.1% respectively). 24 25 KEYWORDS dinoflagellate cysts; earliest Cretaceous (Berriasian); Jurassic; literature 26 analysis and compilation; Triassic; worldwide 27 28 29 1. Introduction 30 The literature on Triassic to earliest Cretaceous (Berriasian) dinoflagellate cysts is extensive, 31 and was listed and reviewed by Riding (2012, 2013, 2014, 2019). -

Ammonite Succession at the Bajocian/Bathonian Boundary in the Cabo Mondego Region
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Ammonite succeSSIon at the Bajocian/Bathonian boundary provided by EPrints Complutense in the Cabo Mondego region (Portugal) SIXTO R. FERNANDEZ-LOPEZ, MARIA HELENA HENRIQUES AND CHARLES MANGOLD The Cabo Mondego outcrops exposed along the cliffs, on the western margin of the Iberian Plate, show an expanded strati graphic section of Lower Bathonian deposits containing abundant ammonoids. Upper Bajocian deposits correspond to similar facies, of muddy limestones alternating with marlstones, although ammonoids are scarce. A detailed succession of ammonites across the Bajocian/Bathonian boundary has been recognized at Cabo Mondego, which can form a useful bio- and chronostratigraphic standard for the Lusitanian Basin. The revision of previous collections from the classical section and new field samplings of two other separate sections allow the recognition through up to twenty metres of thickness, the highest zone of Bajocian (Parkinsoni Zone) and the lowest zone of Bathonian (Zigzag Zone). The Parkinsoni and the Zigzag zones established for NW European areas and belonging to the Northwest European Province, can be identified in the Lusitanian Basin, although the ammonite fossil assemblages are composed of Submediterranean taxa. However, a subdivision of the Parkinsoni Zone is not possible, due to the scarcity of well preserved ammonoids. The Zigzag Zone can be recognized and characterized as composed of two subunits (Parvum and Macrescens subzones) as represented in diverse European basins of the Submedi terranean Province. Ammonite fossil assemblages of the Parvum Subzone may be grouped into two successive horizons, which are biochronostratigraphically equivalent to the subdivisions of the Convergens Subzone distinguished in the Digne-Barreme area (SE France). -

Callovian (Jurassic) Ammonites from the United States and Alaska
Callovian (Jurassic) Ammonites from the United States and Alaska Part 1. Western Interior United States GEOLOGICAL SURVEY PROFESSIONAL PAPER 249-A Callovian (Jurassic) Ammonites from the United States and Alaska Part 1. Western Interior United States By RALPH W. IMLAY GEOLOGICAL SURVEY PROFESSIONAL PAPER 249-A Descriptions and illustrations of cephalopods of Late Jurassic age UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1953 UNITED STATES DEPARTMENT OF THE INTERIOR Oscar L. Chapman, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. CONTENTS Page Page Abstract -—--—---—-——————_—————__________.._____.„__.______ 1 Ecologic considerations—Continued Introduction --——————————————.._____________._„._.„___ 1 Conditions of deposition—— _.____.__———-——.——— 9 Biologic analysis ——————.——__——._____.___.._____„_„_____ 1 Conglomerate —...— ——————————_.., 9 Stratigraphic summary ———————._.__——____.____________ 4 Sand ——_———————————————— — 9 Faunal zones and correlations————..__————_.._——— 5 Marine siltstone and shale.—————-—_——— 10 Arcticoceras codyense zone..————.._______....——_„ 5 Limestone -——————_.-_————————.——— 10 Gowericeras costidensum zone____—._...„——_.——_——— 7 Other types of sediment———————————— 10 Gowericeras subitum zone————_-.........._..._„„........ 7 Ammonite distribution and associations—————— 11 Kepplerites tychonis zone.__————————_———. 7 Geographic distribution - - ———————————————— 14 Kepplerites mcleami zone.—————„___„..—.............. 7 Summary of results--------———————————————— 17 Comparisons with other faunas————____........„—....„.. 8 Systematic descriptions ———.—_.————————— 18 Ecologic considerations —————————_——.__..._.... 8 References ———————————————————— ———. 34 Sources of sediment———_——.._ _„__..—_———._.. 8 Index -.-—————————.———————-—-—--——— 37 ILLUSTRATIONS [Plates 1-24 follow index] PLATE 1. Xenocephalites and Lilloettia 2-4. Arcticoceras 5. Cosmoceras and Arcticoceras 6,7. Cadoceras 8, 9. -

Ammonites from the Latest Aalenian–Earliest Bathonian of La Baume (Castellane Area, SE France): Palaeontology and Biostratigraphy
1661-8726/08/030563-16 Swiss J. Geosci. 101 (2008) 563–578 DOI 10.1007/s00015-008-1297-6 Birkhäuser Verlag, Basel, 2008 Ammonites from the latest Aalenian–earliest Bathonian of La Baume (Castellane area, SE France): palaeontology and biostratigraphy KENNETH DE BAETS 1, 2, *, FABRIZIO CECCA 3, MYETTE GUIOMAR 4 & JACQUES VERNIERS 1 Key words: ammonites, Aalenian–Bathonian interval, France, biodiversity, taxonomy, biostratigraphy ABSTRACT Middle Jurassic strata are naturally exposed around the village called La (Parkinsoni Zone) and probably one lower Bathonian Zone (Zigzag Zone) Baume, near Castellane (Alpes de Haute Provence, SE France). We have re- were found. A major gap of both the Niortense Zone and the Garantiana alized both a detailed log and a bed-by-bed sampling for ammonite biostra- Zone, which was not previously described, was detected at the boundary be- tigraphy in a 68 metres thick succession of subpelagic marls and limestones tween members 2 and 3. The main palaeontological interest of the ammonite (“Calcaires à Zoophycos”) that spans the Middle Jurassic from uppermost fauna from La Baume is the richness and diversity of the family Sonniniidae, Aalenian to lowermost Bathonian. The subpelagic succession can be roughly which is the subject of a systematic study and figurated along with some bio- subdivided into three members. Ammonites from the Upper Aalenian Con- stratigraphically significant forms. Biostratigraphical results and open prob- cavum Zone, all Lower Bajocian Zones (Discites Zone, Laeviuscula Zone lems are discussed. including Ovale Subzone, Humpriesianum Zone), one Upper Bajocian Zone Introduction biostratigraphical subdivisions of the geological formations ex- posed. The Jurassic successions in the area of the Réserve Géologique des Alpes de Haute Provence are widely known because of Geological setting the excellent quality of the exposures, the abundance of am- monoids and the easy access.