1 a Life History Analysis of Invasive Behavior in Native and Naturalized
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Designing Hardwood Tree Plantings for Wildlife Brian J
FNR-213 Hardwood Tree Improvement and Regeneration Center North Central Research Station USDA Forest Service Department of Forestry and Natural Resources Purdue University Designing Hardwood Tree Plantings for Wildlife Brian J. MacGowan, Department of Forestry and Natural Resources, Purdue University Woody plants can be of value to many wildlife species. The species of tree or shrub, or the location, size, and shape of planting can all have an impact on wildlife. The purpose of this paper is to discuss the benefits of trees and shrubs for wildlife and how to design tree and shrub plantings for wildlife. Some of the practices may conflict with other management goals and may have to be modified for individual priorities. Trees and Shrubs for Wildlife The species you select for a tree planting should depend on the growing conditions of the site and the wildlife species that you want to manage. Talk to a professional forester to help you select the tree species best suited for your growing conditions. A professional biologist, such as a Department of Natural Resources District Biologist (www.in.gov/ food source for wildlife (Table 2). Shrubs can be dnr/fishwild/huntguide1/wbiolo.htm), can assist you particularly important because several species of with planning a tree planting for wildlife. wildlife, especially songbirds, prefer to feed or nest There is no specific formula for developing wild- on or near the ground. Shrubs also provide good life habitat. For example, acorns are eaten by a wide protective cover for these types of wildlife. Pines variety of wildlife species including tree squirrels, and other softwoods provide limited food, but are an pheasants, wild turkey, and deer. -

2020 Plant List Index: Trees & Shrubs Pg
2020 Plant List Index: Trees & Shrubs pg. 2-7 Perennials pg. 7-13 Grasses pg. 14 Ferns pg. 14-15 Vines pg. 15 Hours: May 1 – June 30: Tues.- Sat. 10 am - 6 pm Sun.11 am - 5 pm 3351 State Route 37 West www.sciotogardens.com On Mondays by appointment Delaware, OH 43015 Phone/fax: 740-363-8264 Email: [email protected] Sustainable, earth-friendly growth and maintenance practices: Real Soil = Real Difference. All plants are container-grown in a blend of local soil and compost. Plants are grown outside year-round. They are always in step with the seasons. Minimal pruning ensures a well-rooted, healthy plant. Use degradableRoot Pouch andcontainers. recycled containers to reduce waste. Use of controlled-release fertilizers minimizes leaching into the environment. Our primary focus is on native plants. However, non-invasive exotics are an equally important part of the choices we offer you. There is great creative opportunity using natives in combination with exotics. Adding more native plants into our landscapes provides food and habitat for wildlife and connections to larger natural areas. AdditionalAdditional species species may may be be available. available. Email Email oror call for currentcurrent availability, availability, sizes, sizes, and and prices. prices. «BOT_NAME» «BOT_NAME»Wetland Indicator Status—This is listed in parentheses after the common name when a status is known. All species «COM_NAM» «COM_NAM» «DESCRIP»have not been evaluated. The indicator code is helpful in evaluating«DESCRIP» the appropriate habitat for a -

Pocket Guide for Western North Carolina Partnership (SACWMP), 2011
DO NOT BUY Invasive Exotic Plant List Produced by the Southern Appalachian Cooperative Weed Management pocket guide for western north carolina Partnership (SACWMP), 2011 Western North Carolina has to offer! offer! to has Carolina North Western ) allegheniensis Rubus do not buy these invasives buy natives or alternatives ( Blackberry Allegheny ) alba Quercus ( Oak White of beautiful native plants that that plants native beautiful of ! Mimosa (Silk Tree) Albizia julibrissin Common Serviceberry (Amelanchier arborea) ) nigra Juglans ( Walnut Black Eastern Eastern Redbud (Cercis canadensis) multitude the enjoy and environment, To use your pocket guide: ) virginiana Diospyros ( Persimmon Flowering Dogwood (Cornus florida) whole the of quality the to Add counts. ) pumila Castanea ( Chinquapin the environment a favor on both both on favor a environment the 1 Print on letter-size paper. Japanese Barberry Berberis thunbergii Mountain Pepperbush (Clethra acuminata) wildlife for great Virginia Sweetspire (Itea virginica) doing are you plants, native planting Spicebush (Lindera benzoin) By habitat. species’ of loss the and 2 Cut along outer black line. are the spread of invasive exotic plants plants exotic invasive of spread the are ) fistulosum Eupatorium Butterfly Bush Buddleia davidii Swamp Milkweed (Asclepias incarnata) ( Weed Pye Joe ) ) purpurea (Echinacea Coneflower Purple Purple Coneflower (Echinacea purpurea) Carolina North Western in problems 3 Fold on dotted blue lines. ) syriaca Asclepias ( Milkweed Common Joe Pye Weed (Eupatorium fistulosum) -

Vascular Plants for High Park and the Surrounding Humber Plains, 2008
Annotated Checklist of the Vascular Plants for High Park and the Surrounding Humber Plains September 2008 Steve Varga Ontario Ministry of Natural Resources Aurora District Acknowledgements The Ministry has undertaken biological inventories of High Park and its environs over the past 32 years. The first botanical survey was carried out by Karen L. McIntosh as part of an ecological survey of High Park that focussed on Grenadier Pond and the surrounding uplands (Wainio et al. 1976). Botanical surveys were also carried out by the author in 1980, 1982 and 1988. In 1989, this survey information was put together into an Area of Natural and Scientific Interest (ANSI) inventory report of the Park that recognized the natural areas of High Park as a provincial life science ANSI. Subsequent botanical surveys were carried out by the author between 1997 and 2008. Botanical records from High Park have also been kindly provided by Dr. Paul. M. Catling, Dr. Paul F. Maycock, Roger Powley, Charles Kinsley, Gavin Miller, Bohdan Kowalyk, Diana Banville and other members of the Toronto Field Naturalists (TFN), City of Toronto Parks, Forestry & Recreation Division including present and former staff such as Terry Fahey, Cara Webster and Richard Ubens among others, the High Park Community Advisory Committee and it Natural Environment Committee chaired by Karen Yukich, and James Kamstra who in 2007 and 2008 is mapping the location and numbers of rare plants in the Park. The Vascular Plant Herbarium (TRT) at the Royal Ontario Museum and the University of Toronto, Erindale College Herbarium (TRTE) were also examined for historical and recent records from the Park and its surrounding areas. -

Streptopus Lanceolatus (Rosy Twisted-Stalk)
Streptopus lanceolatus Rosy Twisted-Stalk Liliaceae Streptopus lanceolatus by Rob Routledge (CC BY 3.0) Streptopus lanceolatus Rare Plant Profile New Jersey Department of Environmental Protection Division of Parks and Forestry New Jersey Forest Service Office of Natural Lands Management New Jersey Natural Heritage Program 501 East State Street P.O. Box 420 Trenton, NJ 08625-0420 Prepared by: Rebekah Buczynski [email protected] August 21, 2019 This report should be cited as follows: Buczynski, Rebekah. 2019. Streptopus lanceolatus Rare Plant Profile. New Jersey Department of Environmental Protection, Division of Parks and Forestry, New Jersey Forest Service, Office of Natural Lands Management, New Jersey Natural Heritage Program, Trenton, NJ. 15 pp. Streptopus lanceolatus Rare Plant Profile, Page 2 of 15 Introduction Rosy Twisted-stalk is a NJ state endangered plant whose name can arguably be attributed to either its zigzag stem (Peterson and McKenny 1968 and Lady Bird Johnson Wildflower Center 2019 [hereafter, "LBJWC"]) or perhaps more accurately the arching angle of the flowering stalks (LBJWC 2019; Minnesota Wildflowers 2019 [hereafter, "MNWF"]). The translation of the Latin genus Streptopus is literally "Twisted foot" (USDA U.S. Forest Service 2019). One of the common names, "Scootberry" possibly refers to the purgative effect of consuming too much of the fruit (Flowering Plants in Voyageur Country 2007). Another point of taxonomic contention with this species is that Streptopus lanceolatus may be divided into several subordinate taxa depending upon where it exists in its range (MNWF 2019) but for the purposes of this profile we will simply refer to all variations as Streptopus lanceolatus. -

Corydalis Flavula (Raf.) DC
Corydalis flavula (Raf.) DC. yellowyellow fumewort fumewort, Page 1 State Distribution Photo by Bradford S. Slaughter Best Survey Period Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec Status: State threatened capsules containing black, shining seeds. Global and state rank: G5/S2 Range: Yellow fumewort is widespread in the eastern and central United States and Canada, where the species Other common names: yellow corydalis; yellow occurs from Rhode Island south to Florida, west to harlequin Michigan, Illinois, Iowa, and South Dakota, and south to Oklahoma, Arkansas, and Louisiana (Gleason and Family: Papaveraceae (poppy family) Cronquist 1991). The species is considered rare in Connecticut, Delaware, Georgia, Nebraska, New Jersey, Synonyms: Capnoides flavulum(Raf.) �unt�e; Fumaria New York, and Ontario (NatureServe 2009). flavula Raf. State distribution: Yellow fumewort is restricted Taxonomy: The Papaveraceae is a family of ca. 660 to southwestern Lower Michigan, where the species species of herbaceous plants with watery or colored, is known from 17 occurrences in Berrien, Cass, acrid sap, pinnately lobed leaves, 2- or 3-merous �alama�oo, and Calhoun counties. hypogynous flowers, biseriate corollas commonly with 4 or 6 petals, capsular fruits with arillate seeds, and Recognition: Yellow fumewort is a small, semi- the presence of various alkaloids within laticifers or succulent, spreading or sprawling annual forb to secretory cells (Zomlefer 1994). The genus Corydalis is 30 cm tall. The species is characteri�ed by green to often segregated into the family Fumariaceae, which is glaucous, cauline, alternate, bipinnately dissected recogni�ed by some botanists as a distinct family on the leaves and axillary racemes with few to 10 or more basis of several morphological characteristics that often, irregular flowers with 4 unequal yellow petals. -

The Writings of Eloise Butler
The Writings of Eloise Butler A Collection of Garden Experiences - c1916 Mistress Mary, so contrary How does your garden grow? Like Mistress, like garden is the reply. In quirks, in whimsies, and in sheer contrariness a wild garden surpasses Mistress Mary. This is true especially of the introduced species. Last summer a robust specimen of Aster multiflorus [Symphyotrichum ericoides] appeared in the marsh, although it had been placed where it ought to be contented when transplanted from the dry prairie. Gentiana andrewsii has been naturalized by the brook, and now it comes spontaneously on the dry hillsides. Viola conspersa [Viola labradorica - American Dog Violet] was found in large masses putting to shame carefully nurtured specimens planted at the opposite end of the swamp. The showy Liatris pycnostachya has chosen to appear of itself in the meadow, and the little twayblade, Liparis Loeselii, has established itself at a distance from the planted colony. The royal fern, Osmunda regalis, not indigenous to the garden, as was supposed, but laboriously dug and transported from miles sway to the American Dog Violet (Viola labradorica). Photo ©Mark borders of the swamp, has mysteriously sprung up in the center. The Fieder, Wisconsin Flora. most superb growth of Orchis spectabilis [Galearis spectabilis - Showy Orchis] is also unaccounted for, in somewhat dry and infertile soil, where no gardener would ever think of placing it. Castilliea coccina [Scarlet Indian Paintbush], suspected of root parasitism, and accordingly lifted in large blocks of sod, rewarded repeated efforts last season with a single stalk; but at the same time another specimen was found in a seemingly unsuitable place. -

Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments
U.S. Fish & Wildlife Service Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments Contributors: Printing was made possible through the generous funding from Adkins Arboretum; Baltimore County Department of Environmental Protection and Resource Management; Chesapeake Bay Trust; Irvine Natural Science Center; Maryland Native Plant Society; National Fish and Wildlife Foundation; The Nature Conservancy, Maryland-DC Chapter; U.S. Department of Agriculture, Natural Resource Conservation Service, Cape May Plant Materials Center; and U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Reviewers: species included in this guide were reviewed by the following authorities regarding native range, appropriateness for use in individual states, and availability in the nursery trade: Rodney Bartgis, The Nature Conservancy, West Virginia. Ashton Berdine, The Nature Conservancy, West Virginia. Chris Firestone, Bureau of Forestry, Pennsylvania Department of Conservation and Natural Resources. Chris Frye, State Botanist, Wildlife and Heritage Service, Maryland Department of Natural Resources. Mike Hollins, Sylva Native Nursery & Seed Co. William A. McAvoy, Delaware Natural Heritage Program, Delaware Department of Natural Resources and Environmental Control. Mary Pat Rowan, Landscape Architect, Maryland Native Plant Society. Rod Simmons, Maryland Native Plant Society. Alison Sterling, Wildlife Resources Section, West Virginia Department of Natural Resources. Troy Weldy, Associate Botanist, New York Natural Heritage Program, New York State Department of Environmental Conservation. Graphic Design and Layout: Laurie Hewitt, U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Special thanks to: Volunteer Carole Jelich; Christopher F. Miller, Regional Plant Materials Specialist, Natural Resource Conservation Service; and R. Harrison Weigand, Maryland Department of Natural Resources, Maryland Wildlife and Heritage Division for assistance throughout this project. -

Enhancing the Edibility of Landscapes with Native Species
Enhancing the Edibility of Landscapes with Native Species Presented by Russ Cohen at the 2013 RI Land and Water Conservation Summit Saturday, March 9, at the URI Memorial Union, Kingston, RI Flowering Raspberry (Rubus odoratus) in bloom Ripe Butternuts (Juglans cinerea) in their husks •There has been a burgeoning interest in recent years in restoring native plants to our gardens, yards and landscapes (e.g., as evidenced by the 2010 formation of the group Grow Native Massachusetts). •This movement got a major boost several years ago from the publication of the book Bringing Nature Home: How Native Plants Sustain Wildlife in our Gardens. •In Bringing Nature Home, author and University of Delaware Entomology Professor Doug Tallamy makes a compelling case for the key role that native plant species play in supporting our native species of wildlife, particularly insects (such as butterflies and moths), which (in addition to their intrinsic value) serve as a major source of nourishment for nestling birds. Volunteers planting native plant species along the banks of the Housatonic River in Great Barrington, MA A few examples of outreach materials intended to promote and facilitate the planting of native species -- Recommended Native Species for Planting in Lexington, MA National Wildlife Federation’s Community Wildlife Habitat Program Mass. Coastal Zone Management’s Coastal Landscaping with Native Species Excerpt from Rhode Island Coastal Plant Guide – while extremely informative and user-friendly, note the lack of an “edible by humans” column -
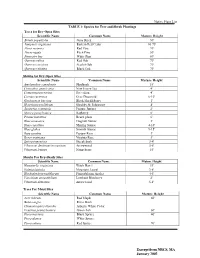
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees For
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees for Dry-Open Sites Scientific Name Common Name Mature Height Betula populifolia Gray Birch 30' Juniperis virginiana Eastern Red Cedar 10-75' Pinus resinosa Red Pine 70' Pin us rigida Pitch Pine 50' Pinus stro bus White Pine 80' Quercus rubra Red Oak 70' Quercus coccinea Scarlet Oak 70' Quercus velutina Black Oak 70' Shrubs for Dry-Open Sites Scientific Name 'Common Name Mature Height Amelanchier canadensis Shadbush 15' Ceanothus americanus New Jersey Tea 4' Comptonia peregrina Sweetfern 4' Cornus racemosa Gray Dogwood 6-10' Gaylussacia baccata Black Huckleberry l' Hypericum prolificum Shrubby St. Johnswort 4' Juniperus communis Pasture Juniper 2' Myrica pensylvanica Bayberry 6' Prunus maritima Beach plum 6' Rhus aromatica Fragrant Sumac 3' Rhus copallina Shining Sumac 4-10' Rhus glabra Smooth Sumac 9-15' Rosa carolina Pasture Rose 3' Rosa virginiana Virginia Rose 3' Spirea tomentosa Steeplebush 3-4' Viburnum dentatum/recognitum Arrowwood 5-8' Viburnum lentago Nannyberry 15' Shrubs For Dry-Shady Sites Scientific Name Common Name Mature Height Hamamelis wrginiana Witch Hazel 15' Kalmia latifolia Mountain Laurel 3-8' Rhododendron nudiflorum Pinxterbloom Azalea 4-6' Vaccinium angustifolium Lowbush Blueberry 2' Viburnum dentatum Arrowwood 5-8' Trees For Moist Sites Scientific Name Common Name Mature Height Acer rubrum Red Maple 60' Betula nigra River Birch Chamaecyparis thyoides Atlantic White Cedar Fraxinus pennsylvanica Green Ash 60' Picea mariana Black Spruce 40' Picea -

Deer Browsing Overwhelms Extended Leaf Phenology Benefits: a Test Case
Forest Ecology and Management 448 (2019) 294–299 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Deer browsing overwhelms extended leaf phenology benefits: A test case with Rubus allegheniensis and a recalcitrant hay-scented fern layer T ⁎ Alejandro A. Royoa, , John S. Stanovickb a USDA Forest Service, Northern Research Station, 335 National Forge Road, P.O. Box 267, Irvine, PA 16329, USA b USDA Forest Service, Northern Research Station, 1549 Long Pond Road, Long Pond, PA 18334, USA ARTICLE INFO ABSTRACT Keywords: Plant species coexistence can be promoted by multiple tradeoffs including temporal niche separation via dif- Odocoileus virginianus ferences in phenology. Namely, if phenological differences afford longer leaf life-spans that confer species the Phenology opportunity to exploit light and fix carbon during periods relatively free of other competitors, then coexistence, Competition or even competitive superiority, may arise. Phenological niche separation explanations, including the Extended Allegheny hardwoods Leaf Phenology (ELP) hypothesis, have garnered considerable support as a mechanistic explanation for both exotic invasive species success and maintenance of native plant species. The benefits conferred by a phenological advantage, however, can be nullified if tissue losses from browsing are coincident with this phenological window of opportunity. This study experimentally tests the role of phenological advantage and white-tailed deer (Odocoileus virginianus) browsing, alone and in concert, in structuring coexistence between the native forest shrub Rubus allegheniensis, which possesses an extended phenological window, and the native invasive fern Dennstaedtia punctilobula. Browsing treatments (ambient versus excluded) were factorially crossed with shading treatments (none versus shading timed to eliminate phenological advantage). -

Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration
Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin A project submitted in partial fulfillment of the requirements for the degree of Master of Environmental Horticulture University of Washington 2013 Committee: Linda Chalker-Scott Kern Ewing Sarah Reichard Joshua Chenoweth Program Authorized to Offer Degree: School of Environmental and Forest Sciences Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin Master of Environmental Horticulture candidate School of Environmental and Forest Sciences University of Washington, Seattle September 3, 2013 Contents Figures ................................................................................................................................................................. ii Tables ................................................................................................................................................................. vi Acknowledgements ....................................................................................................................................... vii Introduction ....................................................................................................................................................... 1 Bromus tectorum L. (BROTEC) ..................................................................................................................... 19 Cirsium arvense (L.) Scop. (CIRARV)