2012 Citizenship & Sustainability Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Health for Humanity Report
2019 Health for Humanity Report Progress in Sustainability Report Summary Contents Message from Our Chairman and CEO 3 Sustainability Approach 4 2019 Year in Brief 5 Better Health for All: Tackling the World’s 6 Toughest Health Challenges Better Health for All: Access, Community 7 Health & Innovation Responsible Business Practices 8 Environmental Health 9 UNICEF, the Government of Vietnam and Johnson & Johnson are partnering on a national program to train more than 500 ethnic minority midwives in remote regions to provide effective maternal and child health interventions including early essential newborn care in village clinics and homes. Photo by Paul Bettings Front cover Volunteers, frontline health workers and government officials at the launch of the Umurinzi vaccination program in Rwanda. In October 2019, Johnson & Johnson committed to donating up to 700,000 regimens of Janssen’s investigational Ebola vaccine to support the Ebola outbreak response in Rwanda and the Democratic Republic of the Congo. Photos by Rwanda Ministry of Health 2019 Health2017 for Health Humanity for HumanityReport Summary Report 33 Message from Our Chairman and CEO Dear Johnson & Johnson Stakeholders, We know this mission will always be unfinished, and affirmed unequivocally that there is a fundamental that we will occasionally fall short. But that only serves connection between serving all stakeholders and 2019 was a year of profound change and great contrasts as motivation to move faster than we’ve ever moved generating sustainable, long-term value. around the globe. before in making bigger strides toward some of our most ambitious goals. And as we’ve detailed in this Report, we The demands for global healthcare and responsible We saw unprecedented innovation and encouraging have plenty of positive momentum worth recognizing. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -
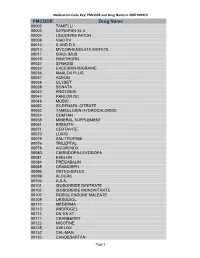
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Frequently Asked Questions
Frequently Asked Questions Why are these companies included on the "Do Test" list? The following companies manufacture products that are tested on animals at some stage of development. Those marked with a Ƈ are currently observing a moratorium on (i.e., current suspension of) animal testing. Please encourage them to announce a permanent ban. Listed in parentheses are examples of products manufactured by either the company listed or, if applicable, its parent company. For a complete listing of products manufactured by a company on this list, please visit the company's website or contact the company directly for more information. Companies on this list may manufacture individual lines of products that have not been tested on animals. They have not, however, eliminated tests on animals for their entire line of cosmetics and household products. What if a company isn't on either of PETA's lists? Some companies have refused to respond to specific questions about their testing practices. It appears likely that these companies do test on animals at some stage of product development, and their refusal to clarify their testing policies appears to be an attempt to mislead consumers. Other companies may be new. If you find a company not included on our lists, please share the company's contact information with PETA so that we can contact the company directly. Legend Ƈ The company is currently observing a moratorium on animal testing. 3M 3M Corporate 1-888-364-3577 www.solutions.3m.com Headquarters 3M Center St. Paul, Minnesota 55144-1000 Acuvue (Johnson & Johnson) Customer (800) 843-2020 www.acuvue.com/ Relations, D-QA 7500 Centurion Parkway Jacksonville, Florida 32256 Aim (Church & Dwight) P.O. -

Cosmetic Formulation of Skin Care Products.Pdf
DK9685_half-series-title 4/25/06 4:34 PM Page A Cosmetic Formulation of Skin Care Products DK9685_half-series-title 4/25/06 4:34 PM Page B COSMETIC SCIENCE AND TECHNOLOGY Series Editor ERIC JUNGERMANN Jungermann Associates, Inc. Phoenix, Arizona 1. Cosmetic and Drug Preservation: Principles and Practice, edited by Jon J. Kabara 2. The Cosmetic Industry: Scientific and Regulatory Foundations, edited by Norman F. Estrin 3. Cosmetic Product Testing: A Modern Psychophysical Approach, Howard R. Moskowitz 4. Cosmetic Analysis: Selective Methods and Techniques, edited by P. Boré 5. Cosmetic Safety: A Primer for Cosmetic Scientists, edited by James H. Whittam 6. Oral Hygiene Products and Practice, Morton Pader 7. Antiperspirants and Deodorants, edited by Karl Laden and Carl B. Felger 8. Clinical Safety and Efficacy Testing of Cosmetics, edited by William C. Waggoner 9. Methods for Cutaneous Investigation, edited by Robert L. Rietschel and Thomas S. Spencer 10. Sunscreens: Development, Evaluation, and Regulatory Aspects, edited by Nicholas J. Lowe and Nadim A. Shaath 11. Glycerine: A Key Cosmetic Ingredient, edited by Eric Jungermann and Norman O. V. Sonntag 12. Handbook of Cosmetic Microbiology, Donald S. Orth 13. Rheological Properties of Cosmetics and Toiletries, edited by Dennis Laba 14. Consumer Testing and Evaluation of Personal Care Products, Howard R. Moskowitz 15. Sunscreens: Development, Evaluation, and Regulatory Aspects. Second Edition, Revised and Expanded, edited by Nicholas J. Lowe, Nadim A. Shaath, and Madhu A. Pathak DK9685_half-series-title 4/25/06 4:34 PM Page C 16. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice, edited by Jon J. -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc. -

Propylene Glycol
PROPYLENE GLYCOL Your patch test result indicates that you have a contact allergy to propylene glycol. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching, and fluid-filled blisters. Where is propylene glycol found? Propylene glycol is used as a softening agent, preservative, humectants, and solvent in cosmetics, fragrances, topical medications, soaps and cleansers, hair care products, and deodorants. Propylene glycol is also found in oral treatments as well as many foods. It is also added during the manufacture of many industrial fluids, such as solvents, thinners, antifreeze, other de-icing fluids, desiccants, brake fluids, and polyester resins. How can you avoid contact with propylene glycol? Avoid products that list any of the following names in the ingredients: • Propylene glycol • 1,2-Dihydroxypropane • CASRN: 57-55-6 • Methylethyl glycol • 1,2-Propanediol • 2-Hydroxypropanol • Isopropylene glycol What are some products that may contain propylene glycol? Antiperspirants and Deodorants: • Old Spice High Endurance • Meguiars Vinyl/Rubber Cleaner/Condition • Adidas 24 Hour Deodorant Control Antiperspirant & Deodorant • Pennzoil Roadside Fix A Flat Tire Sealant & • Adidas 24 Hour Fragrance Clear Stick • Old Spice High Endurance Deodorant Flat Preventative Deodorant • Old Spice Red Zone Clear Gel • Rain-X De-Icer (Aerosol) • Adidas Action 3 Tech F • Old Spice Red Zone Deodorant Stick • Slime -

Pg 1 of 189 Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017
Products and Pricing on the Alberta Blue Cross Drug Price List (ABCDPL) Effective April 13, 2017 DIN/ PIN/ NPN Product Description Route Form MFR Unit of Issue Base Price 00002192691 3TC 10 MG/ML ORAL LIQUID ORL LIQ GSK ML 0.3307 00002192683 3TC 150 MG TABLET ORL TAB GSK TAB 5.0507 00002247825 3TC 300 MG TABLET ORL TAB GSK TAB 9.8070 00002162113 5 BENZAGEL 5% TOPICAL (ALCOHOL) GEL TOP ALCGEL CLC G 0.2080 00002166607 5 BENZAGEL 5% TOPICAL LOTION TOP LOT NVC ML 0.1984 00000437999 5% DEXTROSE & 0.45% NACL W 0.15% KCL 20 MEQ INJ INJ DEFAUL BAX ML 0.0030 00000037974 50% DEXTROSE 500 MG/ML INJECTION USP INJ DEFAUL HSP ML 0.5842 00080013172 5-HTP 100 MG CAPSULE ORL CAP VTH CAP 0.2000 00002414570 ABBOTT-CITALOPRAM 10 MG TABLET ORL TAB ABB TAB 0.1432 00002414589 ABBOTT-CITALOPRAM 20 MG TABLET ORL TAB ABB TAB 0.2397 00002414597 ABBOTT-CITALOPRAM 40 MG TABLET ORL TAB ABB TAB 0.2397 00002412942 ABBOTT-CLOPIDOGREL 75 MG TABLET ORL TAB ABB TAB 0.4735 00002414805 ABBOTT-LEVETIRACETAM 250 MG TABLET ORL TAB ABB TAB 0.8000 00002414791 ABBOTT-LEVETIRACETAM 500 MG TABLET ORL TAB ABB TAB 0.9750 00002414783 ABBOTT-LEVETIRACETAM 750 MG TABLET ORL TAB ABB TAB 1.3500 00002414546 ABBOTT-OLANZAPINE ODT 10 MG TABLET ORL DISNTAB ABB TAB 1.2857 00002414538 ABBOTT-OLANZAPINE ODT 5 MG TABLET ORL DISNTAB ABB TAB 0.6434 00002412969 ABBOTT-PANTOPRAZOLE 40 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.3628 00002412985 ABBOTT-QUETIAPINE 100 MG TABLET ORL TAB ABB TAB 0.3295 00002412993 ABBOTT-QUETIAPINE 200 MG TABLET ORL TAB ABB TAB 0.6617 00002412977 ABBOTT-QUETIAPINE 25 MG TABLET ORL TAB ABB TAB 0.1235 00002413000 ABBOTT-QUETIAPINE 300 MG TABLET ORL TAB ABB TAB 0.9656 00002422638 ABBOTT-RABEPRAZOLE 10 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.1204 00002422646 ABBOTT-RABEPRAZOLE 20 MG ENTERIC-COATED TABLET ORL ECT ABB TAB 0.2408 00002414619 ABBOTT-TOPIRAMATE 100 MG TABLET ORL TAB ABB TAB 0.5928 This document is for pricing use only and should not be used to determine benefit status information. -

Ethics and Social Responsibility
Fundamentals of Business, Second Edition Chapter 4 Ethics and Social Responsibility Content for this chapter was adapted from the Saylor Foundation’s http://www.saylor.org/site/textbooks/Exploring%20Business.docx by Virginia Tech under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License. The Saylor Foundation previously adapted this work under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License without attribution as requested by the work’s original creator or licensee. If you redistribute any part of this work, you must retain on every digital or print page view the following attribution: Download this book for free at: http://hdl.handle.net/10919/84848 Lead: Stephen J. Skripak Contributors: Anastasia Cortes, Gary Walton, Anita Walz Digital and Print Production: Corinne Guimont with Robert Browder Alternative Text and Accessibility: Stephanie Edwards, Christa Miller, and Corinne Guimont Selected graphics: Brian Craig Cover design: Trevor Finney Student Reviewers: Jonathan De Pena, Nina Lindsay, Sachi Soni Project Manager/Editor: Anita Walz This chapter is licensed with a Creative Commons Attribution-Noncommercial-Sharealike 3.0 License. Download this book for free at: http://hdl.handle.net/10919/84848 VT Publishing, a division of the University Libraries at Virginia Tech August 2018 4. Chapter 4 Ethics and Social Responsibility Learning Objectives 1. Define business ethics and explain what it means to act ethically in business. 2. Explain why we study business ethics. 3. Identify ethical issues that you might face in business, such as insider trading, conflicts of interest, and bribery, and explain rationalizations for unethical behavior. 4. Identify steps you can take to maintain your honesty and integrity in a business environment. -

Lna 2006 Profiles J.Qxp
1 | Advertising Age | June 26, 2006 SpecialSpecial ReportReport:100 Profiles LEADING NATIONAL ADVERTISERSSupplement SUPPLEMENT June 26, 2006 100 LEADING NATIONAL ADVERTISERS Profiles of the top 100 U.S. marketers in this 51st annual ranking INSIDE TOP 100 RANKING COMPANY PROFILES SPONSORED BY The nation’s leading marketers Lead marketing personnel, ranked by U.S. advertising brands, agencies, agency expenditures for 2005. contacts, as well as advertising Includes data from TNS Media spending by media and brand, Intelligence and Ad Age’s sales, earnings and more for proprietary estimates of the country’s 100 largest unmeasured spending. PAGE 8 advertisers PAGE 10 This document, and information contained therein, is the copyrighted property of Crain Communications Inc. and The Ad Age Group (© Copyright 2006) and is for your personal, non-commercial use only. You may not reproduce, display on a website, distribute, sell or republish this document, or information contained therein, without prior written consent of The Ad Age Group. Are proud to connect you with the leading CMOs See all the interviews at adage.com/point LAUNCHING JUNE 28 © 2006 Crain Communications Inc. www.adage.com 3 | Advertising Age | June 26, 2006 Special Report 100 LEADING NATIONAL ADVERTISERS SUPPLEMENT ABOUT THIS PROFILE EDITION THE 51ST ANNUAL 100 Leading National the Top 100 ($40.13 billion) and for all measured spending in 18 national media, Advertisers Report crowned acquisition- advertisers ($122.79 billion) in the U.S. Yellow Pages Association contributed ladened Procter & Gamble Co. as the top U.S. ad spending by ad category: This spending in Yellow Pages and TNS Marx leader, passing previous kingpen General chart (Page 6) breaks out 18 measured Promotion Intelligence provided free- Motors Corp. -

Lifescan Ethicon
LouiseLouise MehrotraMehrotra ViceVice PresidentPresident InvestorInvestor RelationsRelations ““SafeSafe HarborHarbor”” StatementStatement This presentation may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from the Company’s expectations and projections. Risks and uncertainties include general industry conditions and competition; economic conditions, such as interest rate and currency exchange rate fluctuations; technological advances and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approvals; domestic and foreign health care reforms and governmental laws and regulations; and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Exhibit 99 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2006. Copies of this Form 10-K, as well as subsequent filings, are available online at www.sec.gov, www.jnj.com or on request from the Company. The Company does not undertake to update any forward-looking statements as a result of new information or future events or developments. WilliamWilliam C.C. WeldonWeldon ChairmanChairman ofof thethe BoardBoard && ChiefChief ExecutiveExecutive OfficerOfficer TodayToday’’ss AgendaAgenda